Given their important biological activities, low bioavailability and structural complexity, access to natural glycostructures or mimics is a major challenge in organic chemistry. Glycosylation is a modification present in many natural molecules and in more than half of the proteins. It involves reacting a glycosyl acceptor on a glycosyl donor using a promoter that activates the starting of a leaving group under the appropriate conditions (solvent, temperature, pressure) to form an acetal, selectively creating an α or β bond.
Glycosylation methodologies
Direct glycosylation using β-N-acetyl-D-peracetylated glucosamine was described by our team in the presence of a catalytic amount of iron(III) triflate under microwave irradiation (A). The reaction has also been carried out in flow chemistry, a technique which has many advantages and allows us to solve the problems of scale up related to the microwave device.

We have also developed a new method of activation of glycosyl sulfone (B) with scandium triflate (70 mol%) under microwave activation. The reaction was particularly efficient for the -mannosylation without a neighboring participating group, leading to the corresponding glycosylation adducts in good yields.
Total synthesis of biomolecules and analogs
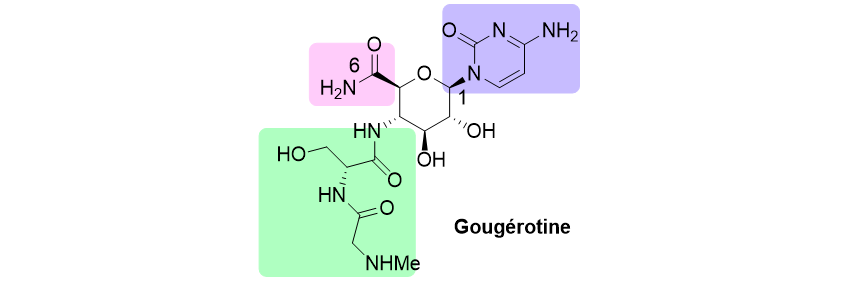
Total synthesis of tiacumicine B, a natural antibiotic
The resistance of bacteria to antibiotics is a real public health problem and one of the solutions to fight against resistance is to develop new molecules, possibly interacting with new biological targets. This is the case of tiacumicin B, a naturally occurring antibiotic that received FDA approval in 2011 in the USA for treatment of the deadly nosocomial diarrhea associated to Clostridium difficile.
This project is being carried out in collaboration with Dr. E. Roulland (University of Paris-Descartes), in charge of the synthesis of the macrolactonic nucleus of the molecule. Our team is developing the chemistry of sugars for the elaboration of rhamnoside (S1) and novioside (S2) fragments, as well as the 1,2-cis glycosylations necessary to link them to the aglycone.
.
Synthesis of molecules iinvolved in plant growth
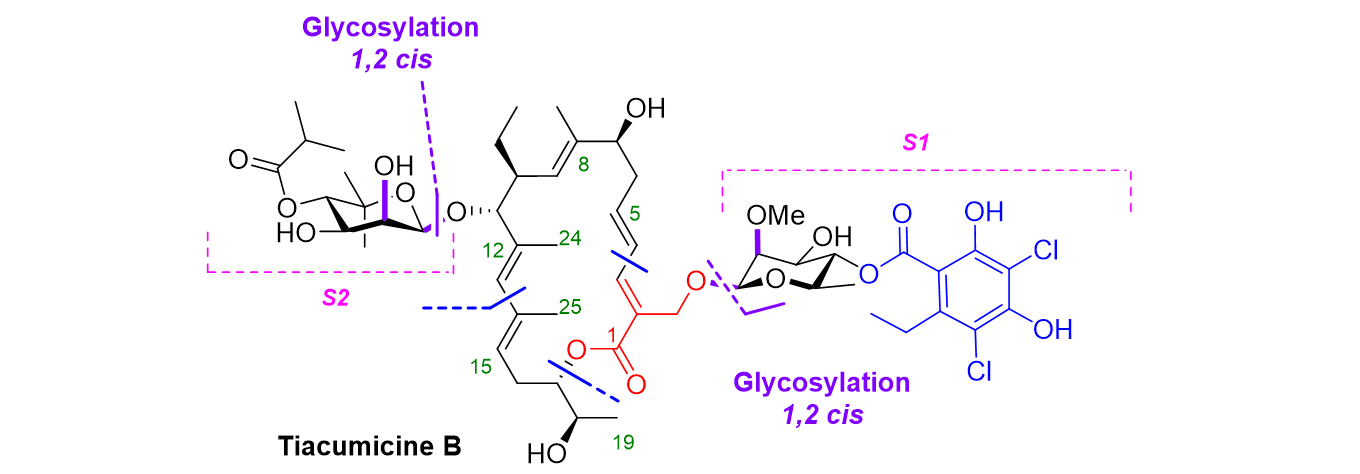
Strigolactones
strigolactones (SLs) are the most recently discovered class of plant hormones. They are involved in controlling the shoot and root plant architecture. This class of molecules includes both canonical and non-canonical SLs, which have a much greater structural variety.
We have so far developed syntheses of canonical SLs and analogues with specific biological activities.
We are currently focusing on the characterization and synthesis of new non-canonical SLs (coll. LBPV University of Nantes, IJPB INRA Versailles, BioCIS Chatenay-Malabry). Given their biological importance, very low bioavailability, complex molecular structures and undefined stereochemistries, these molecules are targets for their total synthesis.
.
Glycolipids analogs
The lipo-chitooligosaccharide (LCO) symbiotic signals are the Nod and LCO Myc factors for rhizobia-legume and arbuscular mycorrhizal symbioses, which are the two main symbioses of ecological importance between higher plants and microorganisms. These molecules perceived by the host plants are necessary for the establishment of a successful association. We are interested in access to these complex structures, which remains a challenge for the glycochemist.

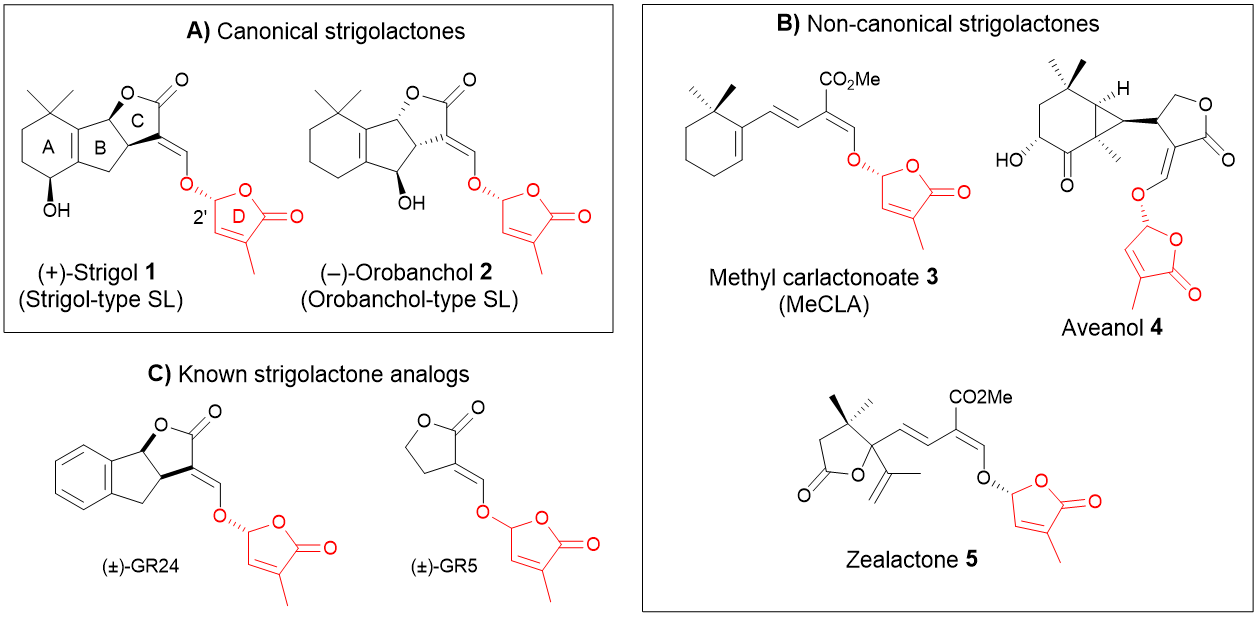
Gourgerotine analogs
We are studying the synthesis of novel analogs of gougerotine in order to study their antifungal and antibacterial activity. These peptido-nucleosides are representatives of a broad class of natural products that inhibit protein synthesis by binding to RNA, resulting in antimicrobial activity. This work proposes to better understand the relationship between the molecule’s substructures and biological activity. The modifications concern more particularly the modification of the nucleic base as well as the replacement of the carboxamide function.