Publications

62. Sulfur-Promoted Synthesis of 2-Aroylquinazolin-4(3H)-ones by Oxidative Condensation of Anthranilamide and Acetophenones
Thanh Binh Nguyen,* Hou Jing-ya, and Pascal Retailleau
Adv. Synth. Catal. 2019
DOI: 10.1002/adsc.201900371
Nguyen, T. B.; Retailleau P. Adv. Synth. Catal. 2019, 10.1002/adsc.201900371
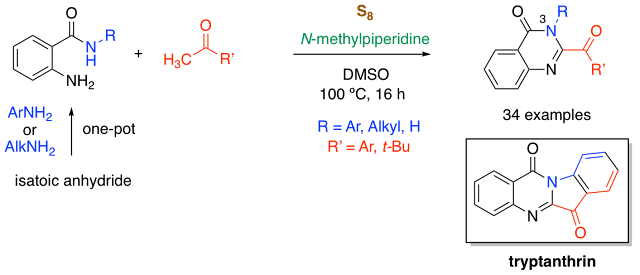
Abstract. A sulfur-promoted three-component reaction of isatoic anhydride, primary aliphatic or aromatic amines, and acetophenones leading to densely substituted 3-substituted 2-aroylquinazolin-4(3H)-ones is reported. The key step involves a cascade reaction of selective oxidation of the methyl group of the acetophenones, followed by a condensation with anthranilamides. The scope of the reaction is applicable to the synthesis of tryptanthrin and various 3-unsubstituted 2-aroylquinazolin-4(3H)-ones.
61. Sulfur‐Catalyzed Stereo and Regioselective Synthesis of Heteropropellanes via Oxidative Condensation of Cyclohexanones with 2‐Aminophenols
Thanh Binh Nguyen* and Pascal Retailleau
Adv. Synth. Catal. 2019
DOI: 10.1002/adsc.201900436
Nguyen, T. B.; Retailleau P. Adv. Synth. Catal. 2019, 10.1002/adsc.201900436

Abstract. Sulfur was found to be an excellent catalyst for the oxidative coupling of cyclohexanones with o-aminophenols using DMSO as oxidant. A wide range of heteropropellanes were obtained with complete regio and stereoselectivities and functional group tolerance.
60. Sulfur-Promoted DABCO-Catalyzed Oxidative Trimerization of Phenylacetonitriles
Thanh Binh Nguyen* and Pascal Retailleau
J. Org. Chem. 2019
DOI: 10.1021/acs.joc.9b00408
Nguyen, T. B.; Retailleau P. J. Org. Chem. 2019, DOI: 10.1021/acs.joc.9b00408
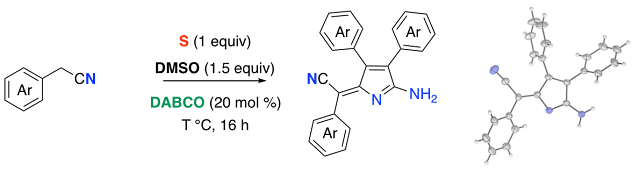
Abstract. By simply heating phenylacetonitriles 1 with elemental sulfur and DMSO in the presence of a catalytic amount of DABCO, we have performed an oxidative trimerization leading to polysubstituted pyrrole heterocycles 2 in excellent yields and E-configuration.
59. Elemental Sulfur/DMSO‐Promoted Multicomponent One‐pot Synthesis of Malonic Acid Derivatives from Maleic Anhydride and Amines
Le Anh Nguyen, Pascal Retailleau and Thanh Binh Nguyen*
Adv. Synth. Catal. 2019
DOI: 10.1002/adsc.201900160
Nguyen, L. A.; Retailleau P.; Nguyen, T. B. Adv. Synth. Catal. 2019, DOI: 10.1002/adsc.201900160
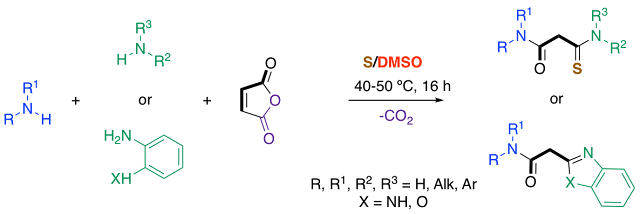
Abstract. Malonic acid derivatives could be conveniently prepared with high degree of functional flexibility via redox condensation reactions between anhydride maleic, amines, elemental sulfur and DMSO as oxidant. This multicomponent decarboxylative transformation consists in a cascade of ring opening, decarboxylative oxidative thioamidation at temperature as low as 50 °C.
58. Sulfur‐Catalyzed Oxidative Coupling of Dibenzyl Disulfides with Amines: Access to Thioamides and Aza Heterocycles
Thanh Binh Nguyen,* Le Phuong Anh Nguyen and Thi Thu Tram Nguyen
Adv. Synth. Catal. 2019
DOI: 10.1002/adsc.201801695
Nguyen, T. B.; Nguyen, L. P. A.; Nguyen, T. T. T. Adv. Synth. Catal. 2019, DOI: 10.1002/adsc.201801695
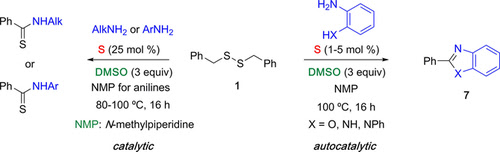
Abstract. In the presence of catalytic amounts of elemental sulfur, dibenzyl disulfide/DMSO was found to be an excellent thiobenzoylating agent of amines to provide a wide range of thioamides. The reaction becomes autocatalytic when anilines substituted by an o‐cyclizable group were used as nucleophile, leading to the corresponding 2‐aryl aza heterocycles.
57. Sulfur-Promoted Decarboxylative Sulfurative Hexamerization of Phenylacetic Acids: Direct Approach to Hexabenzylidyne Tetrasulfides
Thanh Binh Nguyen* and Pascal Retailleau
Org. Lett. 2019, 21, 279
DOI: 10.1021/acs.orglett.8b03728
Nguyen, T. B.; Retailleau, P. Org. Lett. 2019, 21, 279

Abstract. During our experiments aimed at understanding the reaction pathways by which arylacetic acids were oxidatively decarboxylated and condensed with different nucleophiles in the presence of elemental sulfur, these acids have been treated with sulfur powder in dimethyl sulfoxide (DMSO) and N-methylpiperidine in the absence of nucleophiles, producing a remarkable symmetrical sulfurated hexamer consisting of six benzylidyne moieties and four sulfur atoms.
56. Sulfur-Promoted Aminative Aromatization of 1,2,3,4- Tetrahydrophenazines with Amines: Flexible Access to 1- Aminophenazines
Thanh Binh Nguyen* and Pascal Retailleau
Adv. Synth. Catal. 2018, 360, 2389.
DOI: 10.1002/adsc.201800260
Nguyen, T. B.; Retailleau, P. Adv. Synth. Catal. 2018, 360, 2389.
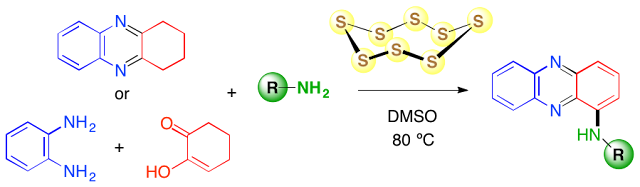
Abstract. Elemental sulfur was found to be an excellent reagent for promoting the aminative aromatization of 1,2,3,4-tetrahydrophenazines with amines to provide a wide range of functionalized 1-aminophenazines.
55. Toward the Synthesis of Sceptrin and Benzosceptrin: Solvent Effect in Stereo- and Regio- Selective [2 + 2] Photodimerization and easy Access to the Fully Substituted Benzobutene System
Thanh Binh Nguyen, Le Anh Nguyen, Mathilde Corbin, Pascal Retailleau, Ludmila Ermolenko, and Ali Al-Mourabit,
Eur. J. Org. Chem. 2018, 5861.
DOI: 10.1002/ejoc.201701607
Nguyen, T. B.; Nguyen, L. A.; Corbin, M.; Retailleau, P.; Ermolenko, L.; Al-Mourabit, A. Eur. J. Org. Chem. 2018, 5861.
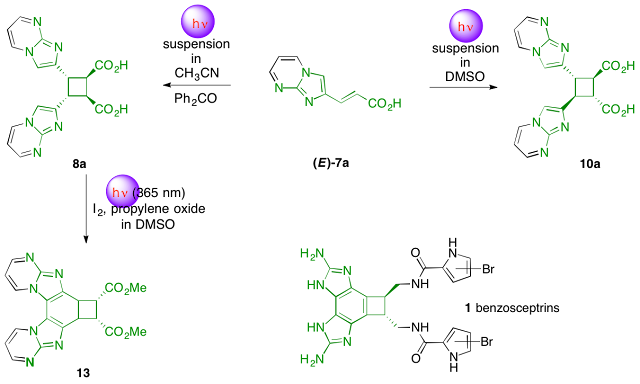
Abstract. By simply switching solvents, two sets of conditions for total regio- and stereo- selective photodimerization of (E)-3-(imidazo[1,2-a]pyrimidin-2-yl)acrylic acid (E)-7a were found. In acetonitrile in the presence of benzophenone as a photosensitizer or in cyclohexane, head-to-head cis-trans-cis isomer 8a was the only observable cycloadduct. In DMSO, head-to-head trans-trans-trans isomer 10a was obtained as the unique cycloadduct. The diester 9a was cyclized into the challenging fully substituted diimidazobenzobutane 13 system present in benzosceptrins.
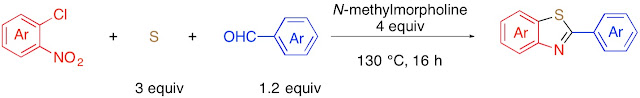
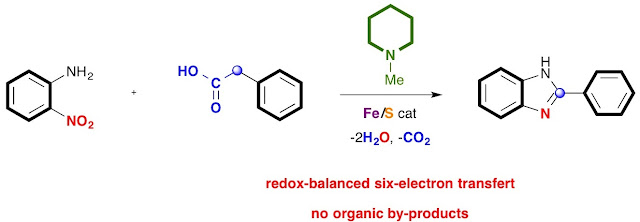
54. Iron-Catalyzed Sulfur-Promoted Decyanative Redox Condensation of o-Nitrophenols and Arylacetonitriles: an Unprecedented Route to 2-Arylbenzoxazoles
Thanh Binh Nguyen* and Jerome Cheung-Lung
Eur. J. Org. Chem. 2018, 5815.
Nguyen, T. B.; Cheung-Lung, J. Eur. J. Org. Chem. 2018, 5815.
DOI: 10.1002/ejoc.201701607

Abstract. Elemental sulfur in the presence of catalytic amount FeCl2•4H2O was found to be highly efficient to promote decyanative redox condensation reactions of o-nitrophenols 1 with arylacetonitriles 2 to a wide range of 2-arylbenzoxazoles 3. The utility of elemental sulfur was highlighted by its role as cyanide scavenger and external reducing agent.
53. Sulfurative Self-Condensation of Ketones and Elemental Sulfur: a Three-Component Access to Thiophenes Catalyzed by Aniline Acid-Base Conjugate Pairs
Thanh Binh Nguyen* and Pascal Retailleau
Green Chem. 2018, 20, 387.
DOI: 10.1039/C7GC03437G
Nguyen, T. B.; Retailleau, P. Green Chem. 2018, 20, 387.
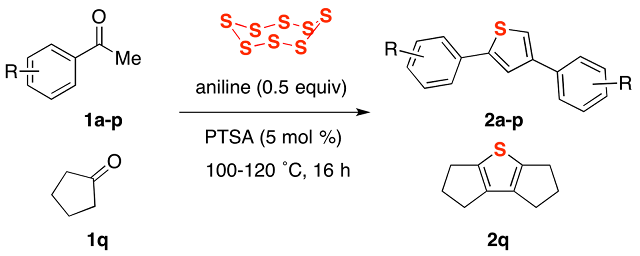
Abstract. A sulfurative self-condensation method for constructing thiophenes 2 by reaction between ketones 1 with elemental sulfur is reported. The reaction, which is catalyzed by anilines and their salt with strong acids, starts from readily available and inexpensive materials, and releases only water as a by-product.
52. Cooperative Activating Effect of Tertiary Amine-DMSO on Elemental Sulfur: Direct Access to Thioaurones from 2’-Nitrochalcones under Mild Conditions
Thanh Binh Nguyen* and Pascal Retailleau
Org. Lett. 2018, 20, 186.
DOI: 10.1021/acs.orglett.ol7b03547
Nguyen, T. B.; Retailleau, P. Org. Lett. 2018, 20, 186.

Abstract. A new mode for the activation of elemental sulfur is reported. In the presence of both DMSO and a tertiary aliphatic amine (triethylamine or N-methylpiperidine), this element reacts directly with a wide range of 2’-nitrochalcones 1 to provide the corresponding thioaurones 2 in high yields even at rt and in the absence of transition metal catalyst.
51. Methyl ketone break-and-rebuild: new strategy for fully α-heterofunctionalization of acetophenones
Thanh Binh Nguyen* and Pascal Retailleau
Green Chem. 2017, 19, 5371.
DOI: 10.1039/C7GC02558K
Nguyen, T. B.; Retailleau, P. Green Chem. 2017, 19, 5371

Abstract. Willgerodt reaction under iron-catalyzed aerobic conditions was found to be an excellent tool for fully α-heterofunctionalization of acetophenones with sulfur and amines. Via this break-and rebuild strategy, a wide range of thioglyoxamides was synthesized in a four-component, highly atom-, step- and redox- economical manner with water as the only by-product.
50. Elemental Sulfur as Reaction Medium for the Synthesis of Fused Nitrogen Heterocycles by Oxidative Coupling between Cycloalkanones and Nitrogen Nucleophiles
Thanh Binh Nguyen* and Pascal Retailleau
Adv. Synth. Catal. 2017, 359, 3843.
DOI: 10.1002/adsc.201700919
Nguyen, T. B.; Retailleau, P. Adv. Synth. Catal. 2017, 359, 3843.
 Abstract. Molten elemental sulfur was found to be an excellent reaction medium for oxidative coupling between bis-aza nucleophiles and cycloalkanones to the fused diaza heterocycles. No extensive aromatization was observed when cyclohexanone was used. These reaction conditions tolerate a wide range of functional groups and are applicable to oxidation sensitive o-phenylenediamines.
Abstract. Molten elemental sulfur was found to be an excellent reaction medium for oxidative coupling between bis-aza nucleophiles and cycloalkanones to the fused diaza heterocycles. No extensive aromatization was observed when cyclohexanone was used. These reaction conditions tolerate a wide range of functional groups and are applicable to oxidation sensitive o-phenylenediamines.
49. DIPEA-Promoted Reaction of 2-Nitrochalcones with Elemental Sulfur: An Unusual Approach to 2-Benzoylbenzothiophenes
Thanh Binh Nguyen* and Pascal Retailleau
Org. Lett. 2017, 19, 4858.
DOI: 10.1021/acs.orglett.7b02321
Nguyen, T. B.; Retailleau, P. Org. Lett. 2017, 19, 4858.
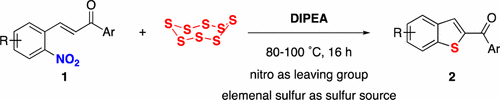
Abstract. DIPEA was found to be an excellent sulfur activator to promote the reaction of 2-nitrochalcones with elemental sulfur. A wide range of 2-benzoylbenzothiophenes was obtained as a result of a cascade of alkene C═C bond thiolation, aromatic sulfur-denitration.
48. Elemental Sulfur as Polyvalent Reagent in Redox Condensation with o-Chloronitrobenzenes and Benzaldehydes: Three-Component Access to 2-Arylbenzothiazoles
Le Anh Nguyen, Anh Quoc Ngo, Pascal Retailleau and Thanh Binh Nguyen*
Green Chem. 2017, 19, 4289.
DOI: 10.1039/C7GC01825H
Nguyen, L. A.; Ngo, Q. A.; Retalleau, P.; Nguyen, T. B. Green Chem. 2017, 19, 4289.
Abstract. Sulfur was found to be a polyvalent reagent in the multicomponent redox condensation with o-chloronitrobenzenes and benzaldehydes. 2-Arylbenzothiazoles was obtained in moderate to good yields in a highly atom-economical pathway.
47. Redox-Neutral Access to Sultams from 2-Nitrochalcones and Sulfur with Complete Atom Economy
Thanh Binh Nguyen,* Pascal Retailleau
Org. Lett. 2017, 19, 3879.
DOI: 10.1021/acs.orglett.7b01766
Nguyen, T. B.; Retailleau, P. Org. Lett. 2017, 19, 3879.
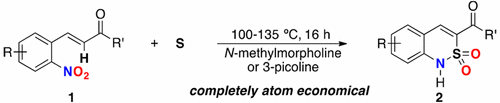
Abstract. A catalyst-free, redox-neutral, and completely atom-economical synthesis of sultams by simply heating 2-nitrochalcones with elemental sulfur in 3-picoline or N-methylmorpholine is described. The S–N, C–S, and S═O bonds of the sulfonamide are efficiently formed between the nitrogen atom of the 2-nitro group and the α-carbon of the chalcones and elemental sulfur with the migration of two oxygen atoms from the 2-nitro group to the sulfur atom.
www.organic-chemistry.org/abstracts/lit5/938.shtm
46. Elemental Sulfur-Promoted Oxidative Rearranging Coupling between o-Aminophenols and Ketones: A Synthesis of 2-Alkylbenzoxazoles under Mild Conditions
Thanh Binh Nguyen,* Pascal Retailleau
Org. Lett. 2017, 19, 3887.
DOI: 10.1021/acs.orglett.7b01775
Nguyen, T. B.; Retailleau, P. Org. Lett. 2017, 19, 3887.
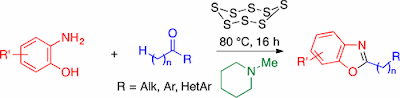
Abstract. In the presence of N-methylpiperidine, elemental sulfur was found to act as excellent oxidant in promoting oxidative rearranging coupling between o-aminophenols and ketones. A wide range of 2-alkylbenzoxazoles was obtained under mild conditions.
www.organic-chemistry.org/abstracts/lit5/941.shtm
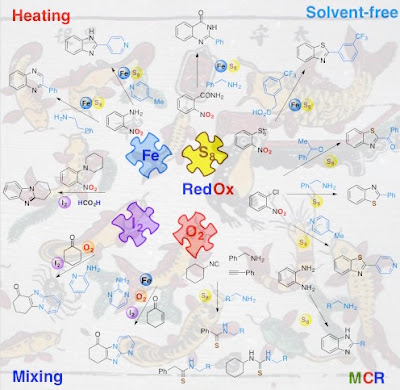
45. Recent Advances in Organic Reactions InvolvingElemental Sulfur
Thanh Binh Nguyen
Adv. Synth. Catal. 2017, 359, 1106.
DOI: 10.1002/adsc.201601329
Nguyen, T. B. Adv. Synth. Catal. 2017, 359, 1106.

Abstract. Elemental sulfur has been known since Antiquity and found widespread applications in the preparation of black gunpowder, the synthesis of sulfuric acid as well as other sulfur-containing compounds, and vulcanization of rubber. Over the last several years, we come to better understand its properties and discover more applications of elemental sulfur in synthetic organic chemistry. This review summarizes the advances from 2000 in the construction of organic molecules using elemental sulfur via sulfuration, oxidation, reduction and redox condensation processes.
1. Introduction
2. Sulfur as Building Block – Sulfuration Reactions
2.1. Sulfuration Reactions with One Substrate
2.2. Sulfuration Reactions with More than One Substrate
3. Sulfur as Oxidant
4. Sulfur as Reductant
5. Sulfur as Catalyst
5.1. Sulfur as the Only Catalyst
5.2. Iron–Sulfur Catalyst for Redox Condensation Reactions of the Nitro Group
6. Conclusion
Keywords: elemental sulfur; organosulfur compounds; cyclooctasulfur; sulfur; sulfuration; sulfur heterocycle
44. Elemental sulfur and molecular iodine as efficient tools for carbon-nitrogen bond formation via redox reactions
Thanh Binh Nguyen
Asian J. Org. Chem. 2017, 6, 477.
DOI: 10.1002/ajoc.201700011
Nguyen, T. B. Asian J. Org. Chem. 2017, 6, 477.
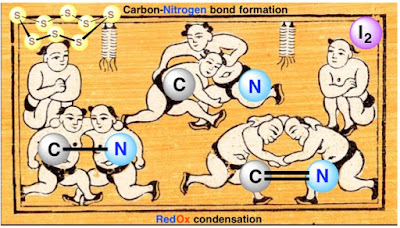
Abstract. Carbon-nitrogen bonds are ubiquitous in organic chemistry. This review will analyze some recent advances in the redox reactions leading to these important linkages involving elemental sulfur and molecular iodine as excellent reagents/catalysts.
43. Remarkably high homoselectivity in [2 + 2] photodimerization of trans-cinnamic acids in multicomponent systems
Thanh Binh Nguyen* and Ali Al-Mourabit
Photochem. Photobiol. Sci. 2016, 15, 1115.
Nguyen, T. B.; Almourabit, A. Photochem. Photobiol. Sci. 2016, 15, 1115.
DOI: 10.1039/C6PP00201C
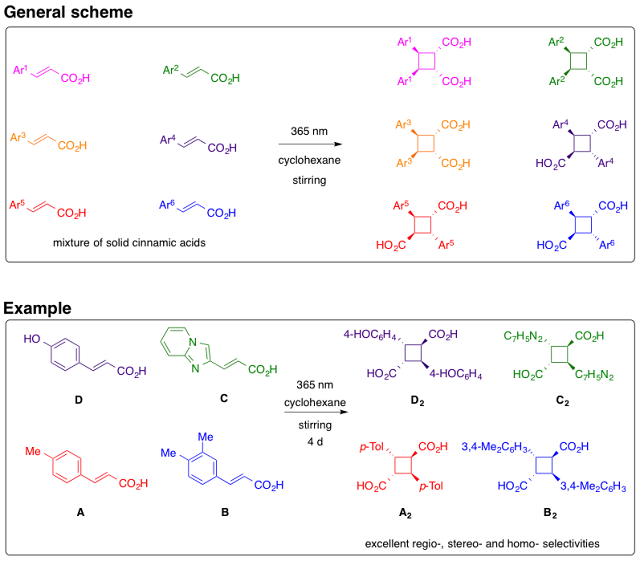
Abstract. [2 + 2] homoadducts were exclusively obtained with total regio- and stereo-selectivities when a suspension of several solid photoactive trans-cinnamic acids in cyclohexane was stirred and irradiated.
42. Molecular Iodine-Catalyzed Aerobic α,β-Diamination of Cyclohexanones with 2-Aminopyrimidine and 2-Aminopyridines
Thanh Binh Nguyen*, Ludmila Ermolenko, Pascal Retailleau, and Ali Al-Mourabit*
Org. Lett. 2016, 18, 2177.
DOI: 10.1021/acs.orglett.6b00823
Nguyen, T. B.; Ermolenko, L.; Almourabit, A. Org. Lett. 2016, 18, 2177.
Abstract. Molecular iodine is shown to be an excellent catalyst for aerobic oxidative α,β-diamination of cyclohexanones with 2-aminopyrimidine/2-aminopyridines. This α,β-C–H functionalization is remarkable for its simplicity in both substrates and conditions, involving one and a half oxygen molecules and releasing three water molecules as the only byproduct. In addition, the functionalized products including protected 2-aminoimidazoles introduced without aromatization can serve as useful building blocks for natural product synthesis and medicinal chemistry.
41. Formic Acid as a Sustainable and Complementary Reductant: Approach to Fused Benzimidazoles by Molecular Iodine-Catalyzed Reductive Redox Cyclization of o-Nitro-t-Anilines
Thanh Binh Nguyen, Ludmila Ermolenko and Ali Al Mourabit
Green Chem. 2016, 18, 2966.
DOI: 10.1039/C6GC00902F
Nguyen, T. B.; Ermolenko, L.; Almourabit, A. Green Chem. 2016, 18, 2966.
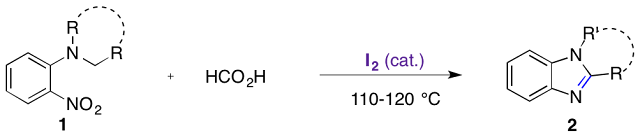
Abstract. Molecular iodine was found to be an excellent catalyst for reductive redox cyclization of o-nitro-t-anilines 1 into fused tricyclic or 1,2-disubtituted benzimidazoles 2. A range of functions such as halides (F, Cl, Br), methoxy, ester, trifluoromethyl, cyano, pyridine and even nitro groups were tolerated using formic acid as a clean, safe, user-friendly and complementary reductant. When iodine was used in stoichiometric amount (50 mol %), the methodology allowed direct synthesis of benzimidazole hydroiodides 2.HI in high yields by simple precipitation from reaction mixture.
40. Redox condensation of o-Halonitrobenzene with 1,2,3,4-Tetrahydroisoquinoline: Involvement of an Unexpected Auto-catalyzed Redox Cascade
Thanh Binh Nguyen, Ludmila Ermolenko and Ali Al Mourabit
Chem. Commun. 2016, 52, 4914.
DOI: 10.1039/C6CC01436D
Nguyen, T. B.;Ermolenko, L.; Almourabit, A. Chem. Commun. 2016, 52, 4914.
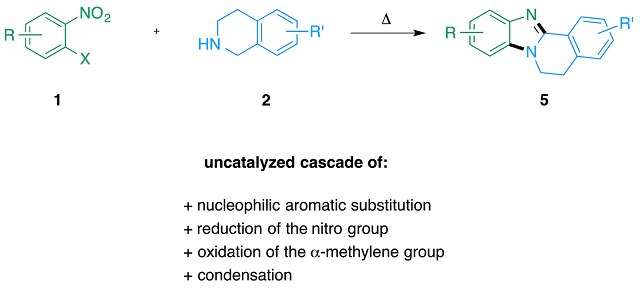
Abstract. A practical synthesis of fused benzimidazoles 5 has been developed by simply heating o-halonitrobenzenes 1 with tetrahydroisoquinolines 2. In this transformation, 2 played multiple roles: building block, base and double hydride donor in a cascade of uncatalyzed aromatic substitution, reduction of the nitro group, oxidation of the α-methylene group and condensation.
39. Vacuum Desiccator as a Simple, Robust, and Inexpensive NMR Tube Cleaner
Thanh Binh Nguyen
Org. Process Res. Dev. 2016, 20, 319.
DOI: 10.1021/acs.oprd.6b00001
Nguyen, T. B. Org. Process Res. Dev. 2016, 20, 319.

Abstract. A simple, robust, and inexpensive apparatus for cleaning several NMR tubes is easily fit up and used from readily available glassware, including a beaker, a desiccator, and a rotary evaporator vacuum pump.

A Spiffy Way To Clean NMR Tubes
Lab Techniques: A simple vacuum setup and a little solvent offer a fast, inexpensive way to clean many glass tubes at once
By Stephen K. Ritter

Life in a chemistry lab isn’t always fun and games—chemists must also do their chores, including cleaning out used NMR tubes. Although commercial devices are available for the task, they are expensive glassware and typically only clean one tube or a few at a time. To remedy that problem, synthetic organic chemist Thanh Binh Nguyen of the Institut de Chimie des Substances Naturelles – CNRS has devised an NMR tube cleaning system that can handle dozens of tubes at once and only requires a small amount of solvent and equipment already at hand in most labs (Org. Process Res. Dev. 2016, DOI: 10.1021/acs.oprd.6b00001). First, Binh empties NMR tubes and places them upside down in a beaker containing solvent or cleaning solution. Binh then places the beaker in a vacuum desiccator, which he evacuates and vents with air several times. The liquid rises and falls with each vacuum cycle, cleaning out the tubes. A final rinse with fresh acetone completes the cleaning. Binh says he came up with the idea when his research funds were short. “I had to optimize everything—time, chemicals, human power—and here is one of my solutions.” Early responses to the OPR&D paper on Twitter were mixed: Some commenters questioned publishing the work in an industrial process chemistry journal, noting that industrial chemists often consider NMR tubes as a one-time consumable and toss them out. Plus cleaning them creates more lab waste. But most admit it’s a clever idea.
38. Elements as Direct Feedstocks for Organic Synthesis: Fe/I2/O2 for Diamination of 2-Cyclohexenones with 2-Aminopyrimidine and 2-Aminopyridines
Thanh BinhNguyen,MathildeCorbin, PascalRetailleau, LudmilaErmolenko, and AliAl-Mourabit
Org. Lett. 2015, 17, 4956.
DOI: 10.1021/acs.orglett.5b02340
Nguyen, T. B.; Corbin, M.; Retailleau, P.; Ermolenko, L.; Almourabit, A. Org. Lett. 2015, 17, 4956.
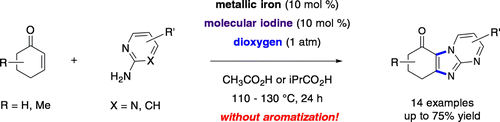
Abstract. Elements as feedstocks for organic synthesis, the trio of metallic iron, molecular iodine, and dioxygen, were found to be an excellent tool for oxidative regioselective diamination of conjugated enones with 2-aminopyrimidine (a guanidine surrogate) and 2-aminopyridines leading to unaromatized coupled products in moderate to good yields.
37. Concise Access to 2-Aroylbenzothiazoles by Redox Condensation Reaction between o-Halonitrobenzenes, Acetophenones, and Elemental Sulfur
Thanh BinhNguyen,KarinePasturaud, LudmilaErmolenko, and AliAl-Mourabit
Org. Lett. 2015, 17, 2562.
DOI: 10.1021/acs.orglett.5b01182
Nguyen, T. B.; Pasturaud, K.; Ermolenko, L.; Almourabit, A. Org. Lett. 2015, 17, 2562.
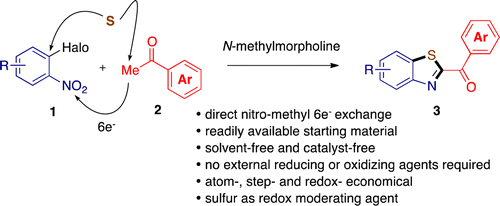
Abstract. A wide range of 2-aroylbenzothiazoles 3 including some pharmacologically relevant derivatives can be obtained in high yields by simply heating o-halonitrobenzenes 1, acetophenones 2, elemental sulfur, and N-methylmorpholine. This three-component nitro methyl coupling was found to occur in an excellent atom-, step-, and redox-efficient manner in which elemental sulfur played the role of nucleophile building block and redox moderating agent to fulfill electronic requirements of the global reaction.
36. Sodium Sulfide: A Sustainable Solution for Unbalanced Redox Condensation Reaction between o-Nitroanilines and Alcohols Catalyzed by an Iron–Sulfur System
Thanh Binh Nguyen,* Ludmila Ermolenko, Ali Al-Mourabit*
Synthesis 2015, 47, 1741.
DOI: 10.1055/s-0034-1380134
Invited Article
Nguyen, T. B.; Ermolenko, L.; Almourabit, A. Synthesis 2015, 47, 1741.
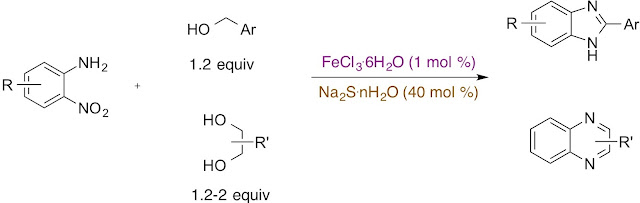
Abstract. Unbalanced redox condensation reaction between o-nitroanilines and alcohols, leading to benzimidazole and quinoxaline heterocycles can be efficiently promoted and catalyzed by sodium sulfide (40 mol%) in combination with iron(III) chloride hexahydrate (1 mol%). Beside the role as a precursor for the iron–sulfur (Fe/S) catalyst formation, hydrated sodium sulfide was shown to be an excellent noncompetitive, multi-electron reducing agent.
35. Elemental Sulfur Disproportionation in the Redox Condensation Reaction between o-Halonitrobenzenes and Benzylamines
Thanh Binh Nguyen, Pascal Retailleau, Ludmila Ermolenko, and Ali Al-Mourabit
Angew. Chem. Int. Ed. 2014, 53, 13808.
DOI: 10.1002/anie.201408397
Nguyen, T. B.; Retailleau, P.; Ermolenko, L.; Almourabit, A. Angew. Chem. Int. Ed. 2014, 53, 13808.

Abstract. The disproportionation of elemental sulfur at moderate temperatures is investigated in the redox condensation involving o-halonitrobenzenes 1 and benzylamines 2. As a redox moderator, elemental sulfur plays the dual role of both electron donor and acceptor, generating its lowest and highest oxidation states: S−2 (sulfide equivalent) in benzothiazole 3 and S+6 (sulfate equivalent) in sulfamate 4, and filling the electron gap of the global redox condensation process. Along with this process, a cascade of reactions of reduction of the nitro group of 1, oxidation of the aminomethyl group of 2, metal-free aromatic halogen substitution, and condensation finally led to 2-arylbenzothiazoles 3.
34. Three-Component Reaction between Isocyanides, Aliphatic Amines and Elemental Sulfur: Preparation of Thioureas under Mild Conditions with Complete Atom Economy
Thanh Binh Nguyen*, Ludmila Ermolenko, Ali Al-Mourabit*
Synthesis 2014, 46, 3172.
DOI: 10.1055/s-0034-1379327
Invited Feature Article
Nguyen, T. B.; Ermolenko, L.; Almourabit, A. Synthesis 2014, 46, 3172.

The reaction of isocyanides with aliphatic amines in the presence of elemental sulfur was found to proceed efficiently at, or near, room temperature to produce thioureas in excellent yields and with complete atom economy.
33. Fe/S-catalyzed decarboxylative redox condensation of arylacetic acids with nitroarenes
Thanh Binh Nguyen,* Ludmila Ermolenko, Mathilde Corbin and Ali Al-Mourabit*
Org. Chem. Front. 2014, 1, 1157.
DOI: 10.1039/C4QO00221K
Nguyen, T. B.; Ermolenko, L.; Corbin, M.; Almourabit, A. Org. Chem. Front. 2014, 1, 1157.
Fe/S clusters generated in situ from simple iron salts and sulfur S8 were found to be highly efficient to catalyze the decarboxylative redox condensation of arylacetic acids with nitroarenes in the presence of N-methylpiperidine as a basic additive. A wide range of aza-heterocycles was obtained in an atom-, step-, and redox-economical manner with water and carbon dioxide as the only by-products.

Cover picture: mice’s wedding Đông Hồ folk woodcut painting (Tranh khắc gỗ dân gian Đông Hồ)
30. Reaction of Quinones and Guanidine Derivatives: Simple Access to Bis-2-aminobenzimidazole Moiety of Benzosceptrin and Other Benzazole Motifs
Thanh BinhNguyen, Minh QuanTran, LudmilaErmolenko, PascalRetailleau, and AliAl-Mourabit
Org. Lett. 2014, 16, 920.
DOI: 10.1021/ol403672p
Nguyen, T. B.; Tran, M. Q.; Ermolenko, L.; Retailleau, P.; Almourabit, A. Org. Lett. 2014, 16, 920.
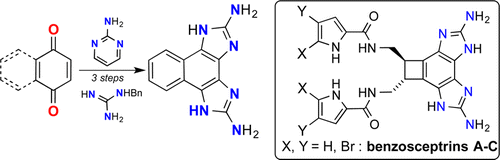
Abstract. A new strategy for the synthesis of 2-aminobenzimidazol-6-ols via a reaction of quinones with guanidine derivatives is reported. Sequential application of this methodology provided a simple access to the first benzosceptrin analogue bearing a bis-2-aminoimidazole moiety. A concomitant addition of two guanidines to the naphtho[1′,2′:4,5]imidazo[1,2-a]pyrimidine-5,6-dione, which includes the redox neutral debenzylation and guanidine-assisted cleavage of the 2-aminopyrimidine part resulted in the synthesis of the free challenging contiguous bis-2-aminoimidazole moiety of benzosceptrins in one step.
29. Three-Component Reaction between Alkynes, Elemental Sulfur, and Aliphatic Amines: A General, Straightforward, and Atom Economical Approach to Thioamides
Thanh BinhNguyen, Minh QuanTran, LudmilaErmolenko, and AliAl-Mourabit
Org. Lett. 2014, 16, 310.
DOI: 10.1021/ol403345e
Nguyen, T. B.; Tran, M. Q.; Retailleau, P.; Almourabit, A. Org. Lett. 2014, 16, 310.
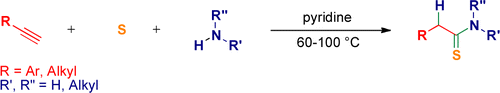
Abstract. A general, straightforward, and atom-economical three-component synthesis of thioamides from alkynes, elemental sulfur, and aliphatic amines has been developed.
28. Cobalt- and Iron-Catalyzed Redox Condensation of o-Substituted Nitrobenzenes with Alkylamines: A Step- and Redox-Economical Synthesis of Diazaheterocycles
Thanh BinhNguyen,* Julie LeBescont, LudmilaErmolenko, and AliAl-Mourabit
Org. Lett. 2013, 15, 6218.
DOI: 10.1021/ol403064z
Nguyen, T. B.; Le Bescont, J.; Ermolenko, L.; Almourabit, A. Org. Lett. 2013, 15, 6218.
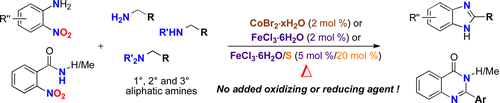
A wide variety of functionalized 2-aryl benzimidazoles can be prepared by a solvent-free cobalt- or iron-catalyzed redox condensation of 2-nitroanilines and benzylamines. The cascade including benzylamine oxidation, nitro reduction, condensation, and aromatization occurs without any added reducing or oxidizing agent. The method can be extended to other alkylamines as reducing components or 2-nitrobenzamides as oxidizing components when using an iron/sulfur catalyst to afford various diazaheterocycles.
27. A Simple and Straightforward Approach to Quinoxalines by Iron/Sulfur-Catalyzed Redox Condensation of o-Nitroanilines and Phenethylamines
Thanh BinhNguyen,* PascalRetailleau, and AliAl-Mourabit
Org. Lett. 2013, 15, 5238.
DOI: 10.1021/ol402435c
Nguyen, T. B.; Retailleau, P.; Almourabit, A. Org. Lett. 2013, 15, 5238.
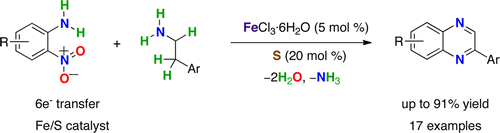
Abstract. In situ generated iron sulfide from elemental sulfur and ferric chloride was found to be a highly efficient catalyst for the redox condensation cascade reaction between o-nitroanilines and 2-arylethylamines. This method constitutes a new atom-, step-, and redox-economical route to 2-arylquinoxalines.
26. Nitro-Methyl Redox Coupling: Efficient Approach to 2-Hetarylbenzothiazoles from 2-Halonitroarene, Methylhetarene, and Elemental Sulfur
Thanh BinhNguyen,* LudmilaErmolenko, and AliAl-Mourabit
Org. Lett. 2013, 15, 4218.
DOI: 10.1021/ol401944a
Nguyen, T. B.; Ermolenko, L.; Almourabit, A. Org. Lett. 2013, 15, 4218.

Abstract. A simple, straightforward, and atom economic approach to 2-hetarylbenzothiazoles starting from 2-halonitroarene, methylhetarene, and elemental sulfur under mild conditions is described. The method is highlighted by the direct redox nitro-methyl reaction for carbon–nitrogen bond formation without an added oxidizing or reducing agent.
Highlighted by Synfacts 2013; 9(11): 1160
25. Selective Autoxidation of Benzylamines: Application to the Synthesis of Some Nitrogen Heterocycles
Thanh Binh Nguyen,* Ludmila Ermolenko and Ali Al-Mourabit
Green Chem. 2013, 15, 2713.
DOI: 10.1039/C3GC41186A
Nguyen, T. B.; Ermolenko, L.; Almourabit, A. Green Chem. 2013, 15, 2713.

Abstract. A green and remarkably simple synthesis of nitrogen heterocycles from benzylamines and anilines o-substituted by a cyclizable group has been developed based on selective uncatalyzed autoxidation of benzylamines.
24. Hydrogen Bond Organocatalysis of Benzotriazole in Transamidation of Carboxamides with Amines
Thanh Binh Nguyen,* Ludmila Ermolenko, Marie-Elise Tran Huu Dau, and Ali Al-Mourabit
Heterocycles, Victor Snieckus’s Special issue, Vol. 88, No. 1, 2014, pp. 403-416
DOI: 10.3987/COM-13-S(S)41
Nguyen, T. B.; Ermolenko, L.; Tran Huu Dau, M.; Almourabit, A. Heterocycles, 2014, 88, 403.
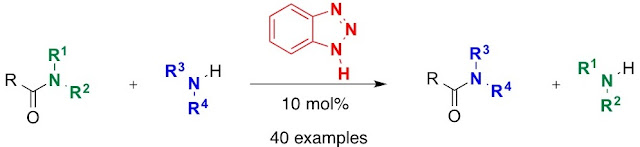
Abstract. A new method of transamidation of carboxamides with amines catalyzed by benzotriazole has been developed.
23. Iron Sulfide Catalyzed Redox/Condensation Cascade Reaction between 2-Amino/Hydroxy Nitrobenzenes and Activated Methyl Groups: A Straightforward Atom Economical Approach to 2-Hetaryl-benzimidazoles and -benzoxazoles
Thanh Binh Nguyen*, Ludmila Ermolenko, and Ali Al-Mourabit
J. Am. Chem. Soc. 2013, 135, 118.
DOI: 10.1021/ja311780a
Nguyen, T. B.; Ermolenko, L.; Almourabit, A. J. Am. Chem. Soc. 2013, 135, 118.

Abstract. Iron sulfide generated in situ from elemental sulfur and iron was found to be highly efficient in catalyzing a redox/condensation cascade reaction between 2-amino/hydroxy nitrobenzenes and activated methyl groups. This method represents a straightforward and highly atom economical approach to 2-hetaryl-benzimidazoles and -benzoxazoles.
22. Benzazoles from Aliphatic Amines and o-Amino/Mercaptan/Hydroxyanilines: Elemental Sulfur as a Highly Efficient and Traceless Oxidizing Agent
Thanh Binh Nguyen,* Ludmila Ermolenko, William A. Dean, and Ali Al-Mourabit
Org. Lett. 2012, 14, 5948.
DOI: 10.1021/ol302856w
Nguyen, T. B.; Ermolenko, L.; Dean, W. A.; Almourabit, A. Org. Lett. 2012, 14, 5948.

Abstract. A novel remarkably simple solvent-free and catalyst-free synthesis of benzazoles from alkylamines and o-hydroxy/amino/mercaptan anilines using elemental sulfur as traceless oxidizing agent has been developed.
Highlighted by Synfact 2013, 148. DOI: 10.1055/s-0032-1318097 Synthesis of Benzazoles Using S8 as the Oxidizing Agent and by http://www.organic-chemistry.org/
21. Efficient and Selective Multicomponent Oxidative Coupling of Two Different Aliphatic Primary Amines into Thioamides by Elemental Sulfur
Thanh BinhNguyen,*LudmilaErmolenko, and AliAl-Mourabit
Org. Lett. 2012, 14, 4274.
DOI: 10.1021/ol3020368
Nguyen, T. B.; Ermolenko, L.; Almourabit, A. Org. Lett. 2012, 14, 4274.

Abstract. An efficient and selective multicomponent oxidative coupling of two different aliphatic primary amines into thioamides by elemental sulfur under solvent-free conditions has been developed.
20. N-Chlorosuccinimide/sodium hydroxide-mediated synthesis of benzimidazoles from amidines under mild conditions
Thanh Binh Nguyen,* Ludmila Ermolenko, and Ali Al-Mourabit
Heterocycles 2012, 86, Prof. Dr. Ei-ichi Negishi’s Special Issue, 555.
DOI: 10.3987/COM-12-S(N)53
Nguyen, T. B.; Ermolenko, L.; Almourabit, A. Heterocycles 2012, 86, 555.
Abstract. A convenient room-temperature one-pot procedure for the preparation of benzimidazoles derivatives from N-arylamidines has been developed. The reaction of N-aryl-N’-chloro amidines, generated by the treatment of N-aryl amidines with N-chlorosuccinimide, in presence of sodium hydroxide provides benzimidazoles in good to excellent yields. Nitrogen anion generated in situ from succinimide (by-product of the chlorination step using NCS) and hydroxide anion was found to be highly effective as Brønsted base to promote the cyclization into benzimidazole of N-aryl-N‘-chloroamidine.
19. Boric Acid: A Highly Efficient Catalyst for Transamidation of Carboxamides with Amines
Thanh BinhNguyen,*Jonathan Sorres, Minh Quan Tran, Ludmila Ermolenko, and Ali Al-Mourabit
Org. Lett. 2012, 14, 3202.
DOI: 10.1021/ol301308c
Nguyen, T. B.; Sorres, J.; Tran, M. Q.; Ermolenko, L.; Almourabit, A. Org. Lett. 2012, 14, 3202.
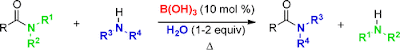
A novel method of transamidation of carboxamides with amines using catalytic amounts of readily available boric acid under solvent-free conditions has been developed. The scope of the methodology has been demonstrated with (i) primary, secondary, and tertiary amides and phthalimide and (ii) aliphatic, aromatic, cyclic, acyclic, primary, and secondary amines.
18. Synthesis, Biological Evaluation, and Molecular Modeling of Natural and Unnatural Flavonoidal Alkaloids, Inhibitors of Kinases
Thanh Binh Nguyen, Olivier Lozach, Georgiana Surpateanu, Qian Wang, Pascal Retailleau, Bogdan Iorga, Laurent Meijer, and Françoise Guéritte
J. Med. Chem. 2012, 55, 2811.

DOI: 10.1021/jm201727w
Abstract. The screening of the ICSN chemical library on various disease-relevant protein kinases led to the identification of natural flavonoidal alkaloids of unknown configuration as potent inhibitors of the CDK1 and CDK5 kinases. We thus developed an efficient and modular synthetic strategy for their preparation and that of analogues in order to determine the absolute configuration of the active natural flavonoidal alkaloids and to provide further insights on the structure–activity relationships in this series. The structural determinants of the interaction between some flavonoidal alkaloids with specific kinases were also evaluated using molecular modeling.
17. Chiral Phosphoric Acid Catalyzed Enantioselective Transfer Hydrogenation of ortho-Hydroxybenzophenone NH Ketimines and Applications
Thanh Binh Nguyen, Qian Wang, and Françoise Guéritte
Chem. Eur. J. 2011, 17, 9576
DOI: 10.1002/chem.201101694
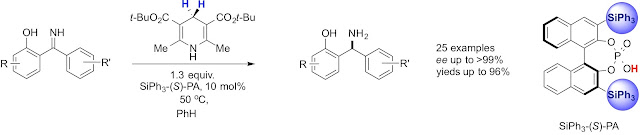
Abstract. Unprotected synthesis: The first enantioselective chiral phosphoric acid catalyzed transfer hydrogenation of unprotected ortho-hydroxybenzophenone NH imines by using a Hantzsch ester as the hydrogen source afforded the corresponding chiral N,O-unprotected ortho-hydroxydiarylmethylamines in high yields with excellent enantioselectivities.
16. Phosphoric Acid-Catalyzed Enantioselective Transfer Hydrogenation of N-Aryl-ortho-Hydroxybenzophenone Ketimines
Thanh Binh Nguyen, Qian Wang, and Françoise Guéritte
Adv. Synth. Catal. 2011, 353, 257.
DOI:10.1002/adsc.201000754
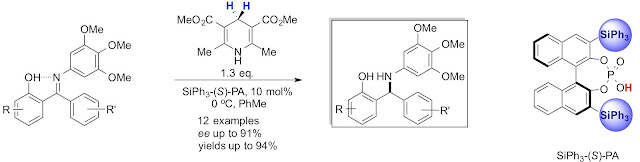
Abstract. The first enantioselective chiral phosphoric acid-catalyzed transfer hydrogenation of N-aryl-ortho-hydroxybenzophenone ketimines using a Hantzsch ester as hydrogen source was developed to afford, after removal of the N-aryl group, the corresponding chiral diarylmethylamines in high yields and enantioselectivities.
15. Chiral Phosphoric Acid-Catalyzed Enantioselective Transfer Hydrogenation of ortho-Hydroxyaryl Alkyl N−H Ketimines
Thanh Binh Nguyen, Hadjira Bousserouel, Qian Wang, and Françoise Guéritte
Org. Lett., 2010, 12 (20), pp 4705–4707
DOI: 10.1021/ol102043x
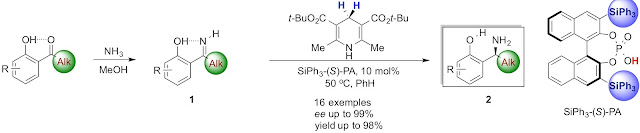
Abstract. The first enantioselective chiral phosphoric acid-catalyzed transfer hydrogenation of unprotected ortho-hydroxyaryl alkyl N−H ketimines using Hantszch di-tert-butyl ester as a reductant is reported. A variety of ortho-hydroxybenzylamines were obtained in good to excellent yields and enantiomeric excesses.
14. An Efficient One-Step Synthesis of Piperidin-2-yl and Pyrrolidin-2-yl Flavonoid Alkaloids through Phenolic Mannich Reactions
Thanh Binh Nguyen, Qian Wang, and Françoise Guéritte
Eur. J. Org. Chem. 2011, 7076.
DOI: 10.1002/ejoc.201101312
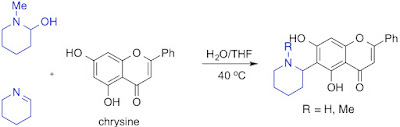
Abstract. An efficient one-step synthesis of piperidin-2-yl and pyrrolidin-2-yl flavonoid alkaloids was achieved in good to excellent yields by a highly regioselective phenolic Mannich reaction of chrysin with cyclic imines or iminium salts. Performing the reaction in a mixture of H2O/THF in the absence of an external reagent afforded the C-6 alkylated product exclusively. The same reaction in water in the presence of NaOH provided the C-8 alkylated flavonoid as a major product. The reaction has been successfully extended to simple phenols.
13. Nguyen, T. B., Wang, Q.; Guéritte, F. Synth. Commun, 2012, 42, 2648. »Practical Synthesis of N-Aryl-o-Hydroxyaryl Ketimines »
Publications prior to postdoc:
12. Nguyen, T. B.; Martel, A.; Dhal, R.; Dujardin, G. Org. Prep. Proc. Int. 2012, 44, 1.
« 1,3-Dipolar Cycloadditions of Nitrones to Hetero-substituted Alkenes Part 2: Sila-, Thia-, Phospha- and Halo-substituted Alkenes »
11. Nguyen, T. B.; Martel, A.; Dhal, R.; Dujardin, G. Org. Prep. Proc. Int. 2010, 42, 387.
“1,3-Dipolar Cycloadditions of Nitrones to Heterosubsituted Alkenes – Part 1: Oxa and Aza-
Substituted Alkenes”
10. Nguyen, T. B.; Martel, A.; Dhal, R.; Dujardin, G. J. Org. Chem. 2010, 75, 611.
“Access to alpha-Substituted Amino Acid Derivatives via 1,3-Dipolar Cycloaddition of alpha-Amino Ester Derived Nitrones”
9. Nguyen, T. B.; Martel, A.; Dhal, R.; Dujardin, G. Synthesis 2009, 3174.
“A Large-Scale Low-Cost Preparation of N-Benzylhydroxylamine Hydrochloride”
Nguyen, T. B.; Martel, A.; Dhal, R.; Dujardin, G. Synlett. 2009, 15, 2492.
« N-(β,β-Difluorovinyl)oxazolidin-2-ones: First Synthesis and Application in [3+2]- and [4+2]-
Cycloaddition-Type Reactions
Nguyen, T. B.; Martel, A.; Dhal, R.; Dujardin, G. Org. Lett. 2008, 10, 4493.
“N-Benzyl Aspartate Nitrones: Unprecedented Single-Step Synthesis and [3 + 2] Cycloaddition Reactions with Alkenes”
6. Nguyen, T. B.; Vuong, T. M. H.; Martel, A.; Dhal, R.; Dujardin, G. Tetrahedron: Asymmetry 2008, 19, 2084.
“Practical Asymmetric Access to Carboxy-Differentiated Aspartate Derivatives via 1,3-Dipolar Cycloaddition of a Nitrone with (R)-4-Ethyl-N-vinyloxazolidin-2-one”
5. Nguyen, T. B.; Martel, A.; Dhal, R.; Dujardin, G. Synlett 2008, 2041.
“Trimethylsilyl Trifluoromethanesulfonate-Mediated Addition-Cyclization of N-Vinyloxazolidin-2- ones to Nitrones: an Efficient Access to 4-Substituted-5-Aza-isoxazolidines”
4. Nguyen, T. B.; Martel, A.; Dhal, R.; Dujardin, G. J. Org. Chem. 2008, 73, 2621.
“1,3-Dipolar Cycloaddition of N-Substituted Dipolarophiles and Nitrones: Highly Efficient Solvent-Free Reaction”
3. Nguyen, T. B.; Gaulon, C.; Chapin, T.; Tardy, S.; Tatibouet, A.; Rollin, P.; Dhal, R.; Martel, A.; Dujardin, G. Synlett 2006, 3255.
“New N-Substituted Dipolarophiles in 1,3-Dipolar Cycloaddition of Nitrones”
1. Nguyen, T. B.; Castanet, A. S.; Nguyen, T. H.; Nguyen, K. P. P.; Bardeau, J. F.; Gibaud, A.; Mortier, J. Tetrahedron 2006, 62, 647.
“Synthesis of Model Long-Chain ω-Alkenyltrichlorosilanes and Triethoxysilanes for the Formation of Self-Assembled Monolayers”
1. Nguyen K. P. P.; Ton, T. Q.; Nguyen, T. B.; Jaureguiberry, G. Vietnamese J. Chem. 2004, 42, 366.
« Some Factors Which Could Induce the Imine – Enamine Tautomerism of Some Schiff Base Adducts of (±)-Gossypol”