Budding yeast S. cerevisiae is an excellent model organism to study genetics, genomics and system biology. Yeast research has also paved the way to understand the molecular basis of many human diseases. Complementary to the functional studies of Fe-S proteins in mammalian cells in our group, we are particularly interested in the yeast Fe-S protein Dre2 that is involved in the biogenesis of the Fe-S clusters.
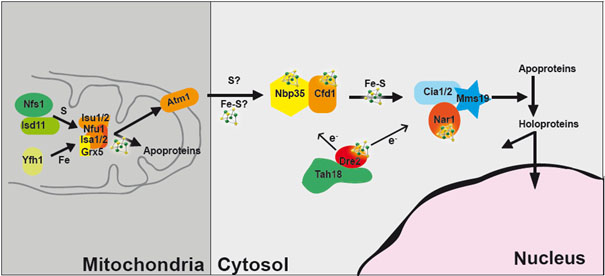
General view of Fe-S biogenesis process in yeast cells. Particular focus is made in our group on the cytosolic Dre2-Tah18 complex.
Notably, Dre2 is the functional human counterpart of CIAPIN1, an antiapoptotic protein involved in many cancers. Dre2 has been the subject of extensive laboratory studies and many molecular tools have already been developed in our group. We identified that Dre2 forms a highly stable, cytosolic protein complex together with the diflavin reductase Tah18. This interaction is essential for cellular viability.
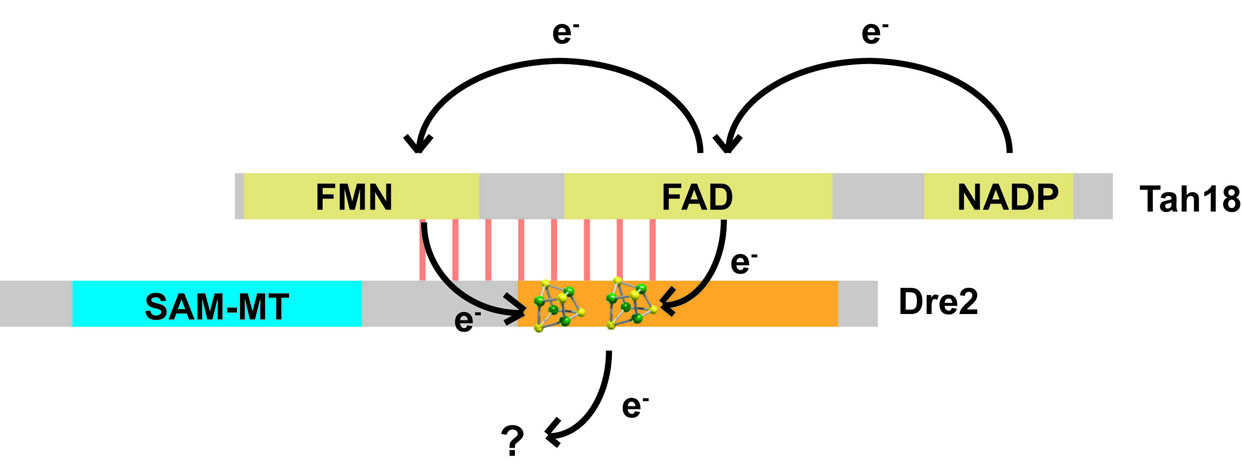
Dre2-Tah18 is a cytosolic electron transfer chain. Potential electron jumps are shown. Questions remains on the destination of electrons from reduced Fe-S in Dre2.
We are currently performing studies to identify new partners of Dre2 that will help better understand the role of this essential protein in several cellular pathways, including cytosolic Fe-S biogenesis, iron homeostasis and oxidative stress response.
A yeast cell drug screening assay based on the Dre2-Tah18 interaction is under development, in order to identity potential new antifungal or anticancer molecules.

