
2022
115. “Divergent cyclodimerizations of styrylnaphthols under aerobic visible-light irradiation and Brønsted acid catalysis » Lyu, J.; Claraz, A.;* Retailleau, P.; Masson, G.* Org. Biomol. Chem. 2022, 20, 9593.

114. “Strategies toward the Difunctionalizations of Enamide Derivatives for Synthesizing α,β-Substituted Amines » Bouchet, D.; Varlet, T.; Masson, G.* Acc. Chem. Res. 2022, 55, 3265.

113. “Enantioselective Construction of Tetrasubstituted Carbon Stereocenters via Chiral Phosphoric Acid-Catalyzed Friedel–Craft Alkylation of Indoles with 5-Substituted Hydroxybutyrolactams » Saidah, M.; Mardjan, M. I. D.; Masson, G.; Parrain, J.-L.; Commeiras, L.* Org. Lett. 2022, 24, 5298.

112. “Decatungstate-Photocatalyzed Dearomative Hydroacylation of Indoles: Direct Synthesis of 2-Acylindolines » Varlet, T.; Bouchet, D.; Van Elslande, E.; Masson, G.* Chem. Eur. J. 2022, e202201707.

111. “Chiral Phosphoric Acid-Catalyzed Enantioselective Formal [4+2] Cycloaddition Between Dienecarbamates and 2-Benzothioazolimines » Ma, W.-Y.; Montinho-Inacio, E.; Iorga, B. I.; Retailleau, P.; Moreau, X.; Neuville, L.; Masson, G.* Adv. Synth. Catal. 2022, 364, 1708.

110. « Recent Advances in C(sp3)–C(sp3) and C(sp3)–C(sp2) Bond Formation through Cathodic Reactions: Reductive and Convergent Paired Electrolyses » Claraz, A.;* Masson, G.* ACS Org. Inorg. Au 2022, doi: 10.1021/acsorginorgau.1c00037.

The formation of C(sp3)–C(sp3) and C(sp3)–C(sp2) bonds is one of the major research goals of synthetic chemists. Electrochemistry is commonly considered to be an appealing means to drive redox reactions in a safe and sustainable fashion and has been utilized for C–C bond-forming reactions. Compared to anodic oxidative methods, which have been extensively explored, cathodic processes are much less investigated, whereas it can pave the way to alternative retrosynthetic disconnections of target molecules and to the discovery of new transformations. This review provides an overview on the recent achievements in the construction of C(sp3)–C(sp3) and C(sp3)–C(sp2) bonds via cathodic reactions since 2017. It includes electrochemical reductions and convergent paired electrolyses.
109. « Electroreductive Cross-Coupling of Trifluoromethyl Alkenes and Redox Active Esters for the Synthesis of Gem-Difluoroalkenes » Claraz, A.;* Allain, C.; Masson, G.* Chem. Eur. J. 2022, doi: 10.1002/chem.202103337.

An electroreductive access to gem-difluoroalkenes has been developed through the decarboxylative/defluorinative coupling of N-hydroxyphtalimides esters and α-trifluoromethyl alkenes. The electrolysis is performed under very simple reaction conditions in an undivided cell using cheap carbon graphite electrodes. This metal-free transformation features broad scope with good to excellent yields. Tertiary, secondary as well as primary alkyl radicals could be easily introduced. α-aminoacids L-aspartic and L-glutamic acid-derived redox active esters were good reactive partners furnishing potentially relevant gem-difluoroalkenes. In addition, it has been demonstrated that our electrosynthetic approach toward the synthesis of gem-difluoroalkenes could use an easily prepared Kratitsky salt as alkyl radical precursor via a deaminative/defluorinative carbofunctionalization of trifluoromethylstyrene.
108. « s-Tetrazine: Robust and Green Photoorganocatalyst for Aerobic Oxidation of N,N-Disubstituted Hydroxylamines to Nitrones » Lyu, J.; Le, T.; Claraz, A.; Allain, C.; Audebert, P.; Masson, G.* Synlett 2022, 33, 177-181

Efficient photocatalytic aerobic oxidative dehydrogenation reactions of N,N-disubstituted hydroxylamines to nitrones were developed with an in situ generated photocatalyst based on commercially available 3,6-dichlorotetrazine. This process affords a wide range of nitrones in high yields under mild conditions. In addition, an oxidative (3+3) cycloaddition between an oxyallyl cation precursor and a hydroxylamine was also developed.
2021
107. « Syntheses of new chiral chimeric photo-organocatalysts » Lyu, J.; Leone, M.; Claraz, A.; Allain, C.; Neuville, L.; Masson, G.* RSC Adv. 2021, 11, 36663-36669.

A new family of chiral chimeric photo-organocatalysts derived from phosphoric acid were synthesized and their spectroscopic and electrochemical properties were investigated. Then, the ability of these photo-activable molecules to catalyse an asymmetric tandem electrophilic β-amination of enecarbamates was evaluated.
106. « Enantioselective and diastereoselective synthesis of spiroindolenines via chiral phosphoric acid-catalyzed cycloaddition » Varlet, T.; Matišić, M.; Van Elslande, E.; Neuville, L.; Gandon, V.;* Masson, G.* J. Am. Chem. Soc. 2021, 143, 16611-16619.

A diastereodivergent and enantioselective synthesis of chiral spirocyclohexyl-indolenines with four contiguous stereogenic centers is achieved by a chiral phosphoric acid-catalyzed cycloaddition of 2-susbtituted 3-indolylmethanols with 1,3-dienecarbamates. Modular access to two different diastereoisomers with high enantioselectivities was obtained by careful choice of reaction conditions. Their functional group manipulation provides an efficient access to enantioenriched spirocyclohexyl-indolines and -oxindoles. The origins of this stereocontrol have been identified using DFT calculations, which reveal an unexpected mechanism compared to our previous work dealing with enecarbamates.
105. « Enamides and dienamides in phosphoric acid-catalysed enantioselective cycloadditions for the synthesis of chiral amines » Varlet, T.; Masson, G.* Chem. Comm. 2021, 57, 4089-4105.

Chiral substituted cyclic amines are ubiquitous among biologically active molecules and natural products and are valuable intermediates in organic synthesis. Stable and easy to synthesize enamides and dienamides are versatile building blocks for the preparation of chiral amines. The exceptional synergy displayed between these synthetic synthons and chiral phosphoric acid catalysts has successfully been harnessed to develop straightforward formal cycloadditions exhibiting notably high enantiocontrol. This feature article showcases the remarkable versatility of these cycloadditions to access chiral cyclic amines with different ring sizes ranging from 5- to 7-membered rings, with an emphasis on biologically active natural products.
104. « Chiral Phosphoric Acid-Catalyzed Enantioselective Construction of 2,3-Disubstituted Indolines« Ma, W.-Y.; Gelis, C.; Bouchet, D.; Retailleau, P.; Moreau, X.; Neuville, L.; Masson, G.* Org. Lett. 2021, 23, 442-448.

Highly enantio- and regioselective (3 + 2) formal cycloaddition of β-substituted ene- and thioenecarbamates as well as cyclic enamides with quinone diimides catalyzed by a BINOL- and SPINOL-derived phosphoric acid is reported. A wide variety of 2,3-disubstituted 2-aminoindolines, including polycyclic ones, were prepared in generally high yields (up to 98%) with moderate to complete diastereoselectivities and in most cases excellent enantioselectivities (up to 99% ee).
105. « Electrochemical tandem trifluoromethylation of allylamines/formal (3 + 2)-cycloaddition for the rapid access to CF3-containing imidazolines and oxazolidines » Claraz, A.;* Djian, A.; Masson, G.* Org. Chem. Front. 2021, 8, 288-296.
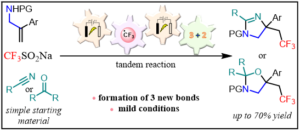
An electrochemical tandem radical trifluoromethylation of allylamines/formal (3 + 2)-cycloaddition with nitrile and carbonyl compounds has been developed under mild and environmentally benign reaction conditions. Such multicomponent reaction allowed the construction of CF3-containing imidazolines and oxazolidines by creating three new bonds from simple and easily available starting materials.
2020
102. « Preparation of Chiral Photosensitive Organocatalysts and Their Application for the Enantioselective Synthesis of 1,2-Diamines » Lyu, J.; Claraz, A.; Vitale, M. R.; Allain, C.; Masson, G.* J. Org. Chem. 2020, 85, 12843-12855.

Chiral phosphoric acid based organocatalysis and visible-light photocatalysis have both emerged as promising technologies for the sustainable production of fine chemicals. In this context, we have envisioned the design and the synthesis of a new class of chimeric catalytic entities that would feature both catalytic capabilities. Given their multitask nature, such catalysts would be particularly attractive for the development of new catalytic transformations, tandem processes in particular. Toward this goal, several BINOL-based chiral phosphoric acid backbones presenting one or two visible-light-sensitive thioxanthone moieties have been prepared and studied. The utility of these new photoactive chiral organocatalysts is then demonstrated in the enantioselective tandem three-component electrophilic amination of enecarbamates. Of note, the C1-symmetric organo/photocatalyst has shown a better catalytic activity than those presenting a C2 symmetry.
101. « La Chimie Organique en France: Une Longue Tradition qui Persiste! » Masson, G.* J. Org. Chem. 2020, 85, 11589-11591.
100. « A Straightforward Synthesis Of A New Family Of Molecules: 2,5,8-Trialkoxyheptazines. Application To Photoredox Catalyzed Transformations » Le, T.; Galmiche, L.; Masson, G.; Allain, C.; Audebert, P.* Chem. Commun. 2020, 56, 10742-10746.

We have prepared several 2,5,8-trialkoxyheptazines starting from the soluble precursor 2,5,8-tris(3,5-diethylpyrazolyl)-heptazine. We present their synthesis along with their promising spectroscopic and electrochemical properties, which demonstrate large band gaps and high reduction potentials altogether. Subsequently, we provide a short assessment of the promising ability of one of these molecules to perform catalytic oxidation test-reactions.
99. « Tritylium Assisted Iodine Catalysis For The Synthesis Of Unsymmetrical Triarylmethanes » Courant, T.; Lombard, M.; Boyarskaya, D. V.; Neuville, L.; Masson, G.* Org. Biomol. Chem. 2020, 18, 6502-6508.

The combined Lewis acid catalytic system, generated from molecular iodine and tritylium tetrafluoroborate effectively catalyzed the Friedel–Crafts (FC) arylation of diarylmethyl sulfides providing an efficient access to various unsymmetrical triarylmethanes. The addition of tritylium and iodine created a more active catalytic system to promote the cleavage of sulfidic C–S bonds.
98. « Selective Double C-H Functionalization: a 4-Component Catellani Reaction » Varlet, T.; Neuville, L.; Masson, G.* Chem 2020, 6, 1855-1858.
Achieving high selectivity through multiple C–H functionalization is a challenging task. In this issue of Chem, Lumb, Luan, and co-workers disclose a clever strategy taking advantage of steric remote effects for the synthesis of poly-substituted arenes through a 4-component Catellani reaction with remarkable regio- and chemoselectivity by using readily available reagents.
97. « Chemical Photocatalysis – Do It Right! » Masson, G.;* Koenig, B.* Eur. J. Org. Chem. 2020, 1191-1192.
96. « Redox‐Divergent Chiral Phosphoric Acid‐Catalyzed Enantioselective Formal Quinone Diels–Alder Reactions » Varlet, T.;§ Gelis, C.;§ Retailleau, P; Bernadat, G.; Neuville, L; Masson, G.* Angew. Chem. Int. Ed. 2020, doi:10.1002/anie.202000838.
§ These authors contributed equally.

An efficient enantioselective construction of tetrahydronaphthalene‐1,4‐diones as well as dihydronaphthalene‐1,4‐diols via a chiral phosphoric acid catalyzed quinone Diels–Alder reaction with dienecarbamate was reported. The nature of the protected group on diene was key to the success showing a remarkable influence for achieving high enantioselectivity. The divergent “redox” selectivity is controlled by adequate amount of quinones used. Reversible redox switching without erosion of enantioselectivity was possible from individual redox isomers.
95. « Electrochemical Intramolecular Oxytrifluoromethylation of N-Tethered Alkenyl Alcohols: Synthesis of Functionalized Morpholines » Claraz, A.;* Courant, T.; Masson, G.* Org. Lett. 2020, 22, 1580-1584.

An electrochemical intramolecular oxytrifluoromethylation of N-tethered alkenyl alcohols was developed providing straightforward access to CF3-containing morpholines derivatives. The method features mild reaction conditions with direct anodic oxidation of Langlois reagent as a cheap and easy to handle trifluoromethylating reagent. Variously substituted 2-(2,2,2-trifluoroethyl)morpholines were obtained in moderate to high yields under constant current electrolysis in an undivided cell.
94. « Enantioselective synthesis of complex fused heterocycles via chiral phosphoric‐acid catalyzed intramolecular inverse‐electron demand aza‐Diels‐Alder reaction » Jarrige, L.; Gandon, V.;* Masson, G.* Chem. Eur. J. 2020, 26, 1406-1413.

A stable asymmetric intramolecular Povarov reaction has been established to provide an efficient method to access structurally diverse trans,trans‐trisubstituted tetrahydrochromeno[4,3‐b]quinolines in high stereoselectivities of up to >99:1 dr and 99% ee, without any purification step. Additionally, in order to facilitate large‐scale application of this method, a low catalyst loading protocol was employed, using 0.2 mol % of chiral phosphoric acid, providing the cycloadducts without any loss in yield and enantioselectivity. The theoretical studies revealed that the reaction occurred through a sequential Mannich reaction and an intramolecular Friedel–Crafts reaction, wherein the phosphoric acid acted as bifunctional catalyst to activate para‐phenolic dienophile and N‐2‐hydroxy‐2‐azadienes, simultaneously.
2019
93. « s-Tetrazine Dyes: a facile generation of photoredox organocatalysts for routine oxidations » Le, T.; Courant, T.; Merad, J.; Allain, C.;* Audebert, P.; Masson, G.* J. Org. Chem. 2019, 84, 16139-16146.

A series of organic dyes derived from s-tetrazine have been synthesized, and their photophysical and electrochemical properties systematically investigated. Testing these compounds as photoredox catalysts in a model oxidative C-S bond cleavage of thi-oethers has led us to identify new classes of active s-tetrazines. Moreover, some of them can be formed in situ from commercially available 3,6-dichlorotetrazine making this photocatalyzed C-S bond functionalization simple and highly practical.
92. « Aerobic tetrazine-catalyzed oxidative nitroso-Diels-Alder reaction of N-arylhydroxylamines with
dienecarbamates: access to functionalized 1,6-dihydro-1,2-oxazines” Le, T.; Courant, T.; Merad, J.; Allain, C.;* Audebert, P.;* Masson, G.* ChemCatChem 2019, 11, 5282-5286.

A new organocatalytic and environmentally friendly aerobic oxidative‐catalyzed nitroso‐Diels‐Alder (NDA) reaction of N‐arylhydroxylamines with dienecarbamates has been developed. 3,6‐Bis(2,2,2‐trifluoroethoxy)‐s‐tetrazine was found to be an effective catalyst to selectively oxidize various N‐arylhydroxylamines in the presence of molecular oxygen, but in the absence of photostimulation, as a terminal oxidant. In addition, active catalytic species can be generated in situ from commercially available 3,6‐dichlorotetrazine.
91. « Combining Organocatalysis and Photoredox Catalysis: An Asymmetric Synthesis of chiral βAmino α-Substituted Tryptamines »Levitre, G.; Audubert, C. Goual, N.; Moreau, X.; Masson, G.* ChemCatChem 2019, 11, 5723-5727.

A stereoselective synthesis of functionalized β‐amino α‐substituted tryptamines has been achieved by a sequential asymmetric organocatalytic three‐component electrophilic amination and photocatalyzed Friedel‐Crafts reaction of indoles with moderate to high diastereoselectivities (up to >99 : 1 dr) and excellent enantioselectivities (up to >99 % ee). We also demonstrated that the β‐amino α‐substituted tryptamines can be successfully engaged in a further catalytic step, furnishing various α,β‐substituted tryptamines without erosion of enantioselectivity and with complete diastereoselectivity.
90. « Four-Component Photoredox-Mediated Azidoalkoxy-trifluoromethylation of Alkenes » Levitre, G.;
Dagousset, G.; Anselmi, E.; Tuccio, B.; Magnier, E.; Masson, G.* Org. Lett. 2019, 21, 6005-6010.

We report herein an efficient four-component photoredox-catalyzed reaction. Under the optimized conditions using [Ru(bpy)3(PF6)2] as the photocatalyst, a wide range of terminal and internal alkenes can efficiently undergo azidoalkoxy-trifluoromethylation in the presence of Umemoto’s reagent, carbonyl compound, and TMSN3, giving rise to original and highly complex molecules in a single operation.
89. « Tandem Chiral Cu(II) Phosphate‐Catalyzed Deoxygenation of Nitrones/Enantioselective Povarov Reaction with Enecarbamates » Gelis, C.; Levitre, G.; Guérineau, V.; Touboul, D.; Neuville, L.; Masson, G.* Eur. J. Org. Chem. 2019, 5151-5159. Cover Feature: Eur. J. Org. Chem. 2019, 4971.

Cut and join together: Chiral Copper phosphate complexes efficiently promoted a tandem sequence of deoxygenation/[4+2] cycloaddition reactions. This process gives access to various tetrahydroquinolines with three consecutive stereogenic centers in good yields with excellent diastereo‐and enantioselectivity.
88. « Chiral Phosphoric Acid-Catalyzed Enantioselective Construction of Structurally Diverse Benzothiazolopyrimidines » Jarrige, L. ; Glavač, D. ; Levitre, G. ; Retailleau, P. ; Bernadat, G. ; Neuville, L. ; Masson, G.* Chem. Sci. 2019, 10, 3765-3769.

A highly efficient catalytic enantioselective [4+2] cycloaddition was developed between 2-benzothiazolimines and enecarbamates. A wide range of benzothiazolopyrimidines bearing three contiguous stereogenic centers was obtained in high to excellent yields and with excellent diastereo- and enantioselectivities (d.r. > 98:2 and up to > 99% ee). Furthermore, this chiral phosphoric acid-catalyzed strategy was scalable and enabled access to a new class of optically pure Lewis base isothiourea derivatives.
87. « Catalyst-free cycloaddition of 1,3-diene-1-carbamates with azodicarboxylates: A rapid click reaction » Varlet, T.;§ Levitre, G.;§ Retailleau, P.; Masson, G.* Bioorg. Med. Chem. 2019, 27, 2438-2443.
§These authors contributed equally.

Novel click reactions are of continued interest in many scientific research areas and applications. Herein, we report a novel practical, catalyst-free, azo-Diels-Alder reaction between dienecarbamates and azodicarboxylates exhibiting a remarkable functional group tolerance. The availability of starting materials, mild reaction conditions, chemoselectivity and scalability make this cycloaddition a viable supplement to the other reactions in “click” chemistry.
2018
86. « Ultrafast Maximum‐Quantum NMR Spectroscopy for the Analysis of Aromatic Mixtures » Concilio, M. G.; Jacquemmoz, C.; Boyarskaya, D.; Masson, G.; Dumez, J. N.* ChemPhysChem. 2018, 19, 3310-3317.

Maximum‐quantum (MaxQ) NMR experiments have been introduced to overcome issues related to peak overlap and high spectral density in the NMR spectra of aromatic mixtures. In MaxQ NMR, spin systems are separated on the basis of the highest‐quantum coherence that they can form. MaxQ experiments are however time consuming and methods have been introduced to accelerate them. In this article, we demonstrate the ultrafast, single‐scan acquisition of MaxQ NMR spectra using spatial encoding of the multiple‐quantum dimension. So far, the spatial encoding methodology has been applied only for the encoding of up to double‐quantum coherences, and here we show that it can be extended to higher coherence orders, to yield a massive reduction of the acquisition time of multi‐quantum spectra of aromatic mixtures, and also to monitor chemical reactions.
85. « Visible light-triggered C-C and C-N bonds formation by C-S bonds cleavage of benzylic thioethers » Lanzi, M.§; Merad, J.§; Boyarskaya, D.V., Maestri, G.; Allain, C.; Masson, G.* Org. Lett. 2018, 20, 5247-5250.
§These authors contributed equally

The cleavage of sulfidic C–S bonds under visible-light irradiation was harnessed to generate carbocations under neutral conditions and synthesize valuable di- and triarylalkanes as well as benzyl amines. To this end, photoredox catalysis and direct photoinduced C–S bond cleavage are used as complementary approaches and participate in the versatility of the general strategy. Extensive mechanistic studies have demonstrated the diversity of the reaction mechanism at work in these different reactions.
84. « Highly Diastereo‐ and Enantioselective Synthesis of Cyclo‐hepta[b]indoles via Chiral Phosphoric Acid‐Catalyzed (4+3)‐Cycloaddition » Gelis, C.§; Levitre, G.§; Merad, J.§; Retailleau, P.; Neuville, L.; Masson, G.* Angew. Chem. Int. Ed. 2018, 57, 12121-12125.
§These authors contributed equally

A highly enantio‐ and diastereoselective formal (4+3) cycloaddition of 1,3‐diene‐1‐carbamates with 3‐indolylmethanols in the presence of a chiral phosphoric acid catalyst is reported. The approach described herein provides efficient access to 6‐aminotetrahydrocyclohepta[b]indoles in good yields with mostly complete diastereoselectivity and excellent levels of enantioselectivity (>98:2 dr and up to 98 % ee). Mild reaction conditions, facile scale‐up, and versatile derivatization highlight the practicality of this methodology. A mechanistic study suggests that cycloaddition occurs in a stepwise fashion, after the formation of an ion pair between the chiral catalytic phosphate and the intermediate carbocation.
83. « Stereoselectivity Switch in the Trapping of Polar Organometallics with Andersen’s Reagent—Access to Highly Stereoenriched Transformable Biphenyls » Levitre, G.; Fer, M. J.; Berreur, J.; Masson, G.; Panossian, A.*; Leroux, F. R.* J. Org. Chem. 2018, 83, 7751-7761.

The trapping of racemic polar carbometallic species with (−)-menthyl (S,S)-p-toluenesulfinate (Andersen’s reagent) typically proceeds with a very low level of resolution. In this paper, we describe a strategy that allows access to highly atropo-enriched and functionalizable biphenyls by means of Andersen’s reagent under kinetic resolution conditions. In particular, useful enantiopure 2-iodobiphenyls could be obtained and were employed in a challenging hypervalent iodine-catalyzed oxidation reaction.
82. « Asymmetric iodine catalysis-mediated enantioselective oxidative transformations » Claraz, A.*; Masson, G.* Org. Biomol. Chem. 2018, 16, 5386-5402.

The implementation of chiral iodine catalysis has tremendously been developed in the field of asymmetric synthesis over the past decade. It enables the stereoselective creation of C–O as well as C–C, C–N and C–X (X = halogen) bonds through oxidative transformations. Thanks to the low toxicity and ease of handling of iodine compounds, this strategy offers many advantages over classical metal-catalyzed oxidations with chiral ligands. The approaches rely on iodine(I/III) or ( −I/+I) catalysis by using a chiral aryliodine or ammonium iodide respectively in combination with a suitable terminal oxidant. As such, the design of iodine compounds with central, axial or even planar chirality has allowed us to achieve high enantioselectivities. The goal of this review is to cover the different chiral iodine compound-catalyzed oxidative transformations including α-functionalization of carbonyl compounds, dearomatization of phenol derivatives and difunctionalization of alkenes which should demonstrate that iodine catalysis has now found its place in the realm of asymmetric organocatalysis.
81. « Author Profile :Géraldine Masson » Masson, G.*Angew. Chem. Int. Ed. 2018, 57, 12208-12209.
80. « L’organocatalyse énantiosélective : moderne, efficace et propre » Jarrige, L.; Masson, G.* Actualité chimique 2018, 426, 63-64. 
L’organocatalyse désigne l’accélération de réactions chimiques par l’ajout d’une quantité substœchiométrique d’un composé organique qui ne contient aucun atome de métal. Elle s’inscrit dans le vaste domaine de recherche de la catalyse, longtemps dominé par la catalyse enzymatique et la catalyse organométallique. Devenue cruciale en synthèse organique, elle s’inscrit parfaitement dans le cadre d’une chimie moderne et respectueuse de l’environnement en maintenant les critères d’efficacité déjà atteints par la catalyse métallo-assistée.
79. « Brønsted Acid Catalysis as a Tool for the Synthesis of Natural Products and Pharmaceuticals » Merad, J.; Lalli, C.; Bernadat, G.; Maury, J.; Masson, G.* Chem. Eur. J. 2018, 24, 3925-3943.

Synthesis of biologically active molecules (whether at laboratory or industrial scale) remains a highly appealing area of modern organic chemistry. Nowadays, the need to access original bioactive scaffolds goes together with the desire to improve synthetic efficiency, while reducing the environmental footprint of chemical activities. Long neglected in the field of total synthesis, enantioselective organocatalysis has recently emerged as an environmentally friendly and indispensable tool for the construction of relevant bioactive molecules. Notably, enantioselective Brønsted acid catalysis has offered new opportunities in terms of both retrosynthetic disconnections and controlling stereoselectivity. The present report attempts to provide an overview of enantioselective total or formal syntheses designed around Brønsted acid-catalyzed transformations. To demonstrate the versatility of the reactions promoted and the diversity of the accessible motifs, this Minireview draws a systematic parallel between methods and retrosynthetic analysis. The manuscript is organized according to the main reaction types and the nature of newly-formed bonds.
2017
78. « Asymmetric α-Sulfonyl- and α-Phosphoryl-Oxylation of Ketones by a Chiral Hypervalent Iodine(III) » Levitre, G. ; Dumoulin, A. ; Retailleau, P. ; Panossian, A. ; Leroux, F. R. ; Masson, G. J. Org. Chem. 2017, 82, 11877-11883.

An enantioselective direct oxygenation of propiophenone derivatives mediated by a catalytic or stoichiometric amount of new chiral non-C2-symmetric iodoarenes(III) is reported. The reaction gives an easy entry to optically active α-sulfonyl- and α-phosphoryl oxyketones in respectable yields and enantioselectivities.
77. « Enantioselective Organocatalytic Intramolecular aza-Diels–Alder Reaction » Jarrige, L. ; Blanchard, F. ; Masson, G. Angew. Chem. Int. Ed. 2017, 56, 10573-10576.

A highly efficient catalytic enantioselective intramolecular Povarov reaction was developed with primary anilines as 2-azadiene precursors. A wide variety of angularly fused azacycles were obtained without column chromatography in high to excellent yields and with excellent diastereo- and enantioselectivity (d.r.>99:1 and up to e.r. 99:1). Furthermore, the catalyst loading could be lowered to 1 mol %, and the obtained azacycles could be used as key intermediates for further transformations to generate additional molecular diversity.
76. « Easy Access to Biologically Active Quinolin-2(1H)-ones via a One-Pot Tandem-oxa-Michael–Aldol Sequence » Jarrige, L. ; Merad, J. ; Zaied, S.; Blanchard, F. ; Masson, G. Synlett 2017, 28, 1724-1728.

An efficient strategy for the synthesis of a variety of quinolin-2(1H)-one derivatives has been developed. The reaction proceeded from cinnamide derivatives via a tandem reaction in the presence of NaOH to afford the corresponding 2- quinolin-2(1H)-one derivatives in good to excellent yields.
75. « Fluorinated Sulfilimino Iminiums: Efficient and Versatile Sources of Perfluoroalkyl Radicals under Photoredox Catalysis » Daniel, M. ; Dagousset, G. ; Diter, P.; Klein, P.-A. ; Tuccio, B. ; Goncalves, A.-M.; Masson, G. ; Magnier, E. Angew. Chem. Int. Ed. 2017, 56, 3997-4001.

Reported herein is the use of S-perfluoroalkyl sulfilimino iminiums as a new source of RF radicals under visible-light photoredox catalysis (RF=CF3, C4F9, CF2Br, CFCl2). These shelf-stable perfluoroalkyl reagents, readily prepared on gram scale from the corresponding sulfoxide using a one-pot procedure, allow the efficient photoredox-induced oxyperfluoroalkylation of various alkenes using fac-Ir(ppy)3 as the photocatalyst. Importantly, spin-trapping/electron paramagnetic resonance experiments were carried out to characterize all the radical intermediates involved in this radical/cationic process.
74. « Enantioselective Three-Component Amination of Enecarbamates Enables the Synthesis of Structurally Complex Small Molecules » Dumoulin, A. ; Bernadat, G. ; Masson, G. J. Org. Chem. 2017, 82, 1775–1789.

The control of asymmetric synthesis tools represents a major challenge, especially when it comes to the synthesis of bioactive molecules. In this context, the asymmetric synthesis of 1,2-diamines through amination of enecarbamates has been proposed as a highly efficient and tunable approach. Indeed, reactivity of the latter species could be exploited to realize a double functionalization via an electrophilic amination followed by nucleophilic trapping. Herein, we describe a chiral phosphoric acid catalyzed electrophilic amination of enecarbamates with dibenzyl azodicarboxylate and oxygenated or thiol-containing nucleophiles affording stable precursors of α-hydrazinoimines in high yields and with almost complete enantioselectivities (up to >99%). These precursors were successfully functionalized with various silylated nucleophiles without epimerization of the stereogenic center, giving access to a wide range of 1,2-disubstituted 1,2-diamines. We also show that the thiolated precursors were successfully engaged in a Friedel–Crafts reaction against a variety of aromatic and heteroaromatic nucleophiles, leading to various 1-(hetero)aryl-1,2-diamines without loss of enantioselectivity and with complete diastereoselectivity. Reductive N–N bond cleavage provided the N,N-diprotected 1,2-diamines with no loss in diastereo- or enantioselectivity. The protocol was successfully scaled up to a multigram scale and the catalyst was successfully recovered, demonstrating the potential applications of this new methodology.
73. « Chiral Hypervalent Iodine(III) Catalyst Promotes Highly Enantioselec-tive Sulfonyloxy- and Phosphoryloxy-Lactonizations » Gelis, C. ; Dumoulin, A. ; Bekkaye, M. ; Neuville, L.; Masson, G. Org. Lett. 2017, 19, 278–281.
 An efficient enantioselective hypervalent iodine promoted oxylactonization of 4-pentenoic acids has been achieved using stoichiometric or a catalytic amount of chiral aryl-λ3-iodane. This reaction provides straightforward access to a wide range of sulfonyloxy- and phosphoryloxy-γ-butyrolactones in respectable yields with moderate to excellent enantioselectivities.
An efficient enantioselective hypervalent iodine promoted oxylactonization of 4-pentenoic acids has been achieved using stoichiometric or a catalytic amount of chiral aryl-λ3-iodane. This reaction provides straightforward access to a wide range of sulfonyloxy- and phosphoryloxy-γ-butyrolactones in respectable yields with moderate to excellent enantioselectivities.
72. « Visible Light Photoredox-Mediated Oxidative Tandem Nitroso Diels-Alder Reaction of Arylhydroxylamines with Conjugated Dienes » Santacroce, V. ; Duboc, R. ; Malacria, M. ; Maestri, G.; Masson, G. Eur. J. Org. Chem. 2017, 2017, 2095–2098.

Arylhydroxylamines were used in the nitroso-Diels–Alder reaction to generate in situ nitrosoarenes under visible-light, catalytic and aerobic conditions. Mixing a solution of aryl- or heteroarylhydroxylamines with conjuguated dienes in the presence of a catalytic amount of Ru(bpy)3Cl2 afforded 3,6-dihydro-1,2-oxazines in good yields under an oxygen atmosphere.
2016
71. « Asymmetric Oxidative Nitroso-Diels–Alder Reaction of N-Arylhydroxylamines Catalyzed by a Chiral Phosphoric Acid” Dumoulin, A.; Masson, G. J. Org. Chem., 2016, 81, 10154–10159.
A highly stereoselective synthesis of cis-3,6-disubstituted dihydro-1,2-oxazines has been accomplished through the one-pot oxidative nitroso-Diels–Alder reaction of N-arylhydroxylamines with diene carbamates. The new system is based on a combination of chiral phosphoric acid and m-CPBA and gives various 3,6-disubstituted dihydro-1,2-oxazines in excellent regio-, diastereo-, and enantioselectivity.
70. « Recent Progress in Visible-Light Photoredox-Catalyzed Intermolecular 1,2-Difunctionalization of Double Bonds via an ATRA-Type Mechanism” Courant T.; Masson, G. J. Org. Chem. 2016, 81, 6945-6952
Radical difunctionalizations of alkenes constitute an efficient method for the construction of complex organic molecules. This synopsis focuses on visible-light catalysis, a recent and very promising technological refinement of this class of transformations. Examples taken from the literature illustrate the use of a variety of (metallic or nonmetallic) systems, which allow us to leverage the energy of readily available visible-light radiation to efficiently create some of the most commonly looked for types of bonds (C–X, C–O, C–N, and C–C) under mild conditions and starting from unsaturated substrates.
69. « Visible-Light Photoredox-Catalyzed Coupling Reaction of Azoles with α-Carbamoyl Sulfides” Jarrige, L. ; Levitre, G.; Masson, G. J. Org. Chem. 2016, 81, 7230-7236.
A simple, straightforward strategy for the synthesis of N-substituted azoles is reported that involves a visible-light photoredox-catalyzed coupling reaction of azoles with α-carbamoyl sulfides. A variety of heterocyclic units, including pyrazoles, benzopyrazoles, benzoimidazoles, and purines, can be efficiently incorporated under mild reaction conditions in respectable yields.
68. « Chiral Phosphoric Acid Catalyzed [3 + 2] Cycloaddition and Tandem Oxidative [3 + 2] Cycloaddition: Asymmetric Synthesis of Substituted 3-Aminodihydrobenzofurans” Gelis, C. ; Bekkaye, M. ; Lebée, C.; Blanchard, F.; Masson, G. Org. Lett. 2016, 18, 3422–3425.
Asymmetric [3 + 2] cycloaddition of quinones with ene- and thioene-carbamates was achieved by chiral phosphoric acid catalysis, providing the corresponding 3-amino-2,3-dihydrobenzofurans in excellent yields with moderate to good diastereoselectivities and excellent enantioselectivities. An asymmetric tandem oxidative cycloaddition protocol starting from hydroquinones was also accomplished with phenyliodine(III) diacetate and a chiral phosphoric acid in the same reaction vessel.
67. « Photoredox-Catalyzed Three-Component Tandem Process: An Assembly of Complex Trifluoromethylated Phthalans and Isoindolines”Jarrige, L. ; Carboni, A. ; Dagousset, G.; Levitre, G.; Magnier, E. ; Masson, G. Org. Lett. 2016, 18, 2906–2909.
A novel photoredox-mediated tandem three-component process afforded a wide variety of CF3-containing phthalans and isoindolines in respectable yields and with moderate to excellent diastereoselectivity.
66. « α -Carbamoylsulfides as N-Carbamoylimine Precursors in the Visible Light Photoredox-Catalyzed Synthesis of α,α-Disubstituted Amines” Lebée, C.; Languet, M.; Allain, C.; Masson, G. Org. Lett. 2016 , 18, 1478–1481.
A novel photoredox-mediated tandem three-component process afforded a wide variety of CF3-containing phthalans and isoindolines in respectable yields and with moderate to excellent diastereoselectivity.
65. » Lewis acids turn unreactive substrates into pure enantiomers » Dumoulin, A. ; Masson, G. Science 2016, 351, 918-919.
Asymmetric synthesis, reactions that produce a high proportion of one enantiomer of a chiral compound relatively to the other, are of ever-growing importance, particularly for creating pure pharmaceuticals. Asymmetric catalysis (1) uses a recyclable or regenerated chiral molecule to perform these reactions, but achieving high yields can be challenging if the substrate is relatively unreactive. On page 949 of this issue, Gatzenmeier et al. (2) report a highly efficient method that relies on an in situ–generated catalyst to produce complex structures in excellent yields of almost pure enantiomers from low-reactivity starting materials.
64. « Highly enantioselective intermolecular iodo- and chloroamination of enecarbamates catalyzed by chiral phosphoric acids or calcium phosphate salts »Lebée, C.; Blanchard, F.; Masson, G. Synlett 2016, 27, 559-563.
Highly enantio- and diastereoselective vicinal chloro- and iodoamination reaction of enecarbamates catalyzed by chiral phosphoric acids or chiral calcium organophosphates are reported. The approach described herein provides efficient access to cis-chloro and cis-iodoaminals in good yields and excellent enantioselectivities (up to 99% ee). The resulting products are converted readily to highly important trans-azidoaminals.
63. « Synthesis of new axially chiral iodoarenes » Bekkaye, M.; Masson, G. Synthesis 2016, 48,302-312.
A new family of axially chiral iodoarenes derived from commercially available (R)-1,1′-binaphthalene-2,2′-diamine has been synthesized and employed as catalysts in Kita’s enantioselective oxidative spirolactonization of propanoic acid-tethered 1-naphthol. Through this study, we explored the relationship between the hypervalent iodoarene geometry and enantioselectivity, contributing to the current understanding of axial-to-central chirality transfer in organoiodine(III) catalysis.
2015
62. « Regio-, diastereo-, and enantioselective nitroso Diels-Alder reaction of 1,3-diene-1-carbamates, catalyzed by chiral phosphoric acids » Pous, J.; Courant, T.; Bernadat, G.; Ioga, B. I.; Blanchard, F.; Masson, G. J. Am. Chem. Soc. 2015, 137, 11950-11953.
Chiral phosphoric acid-catalyzed asymmetric nitroso-Diels–Alder reaction of nitrosoarenes with carbamate-dienes afforded cis-3,6-disubstituted dihydro-1,2-oxazines in high yields with excellent regio-, diastereo- and enantioselectivities. Interestingly, we observed that the catalyst is not only able to control the enantioselectivity but is able to reverse the regioselectivity of the non-catalyzed nitroso-Diels–Alder reaction. Regiochemistry reversal as well as asynchronous concerted mechanism were confirmed by DFT calculations.
61. « Three-component photoredox-mediated chloro-, bromo- or iodo-trifluoro-methylation of alkenes » Carboni, C.; Dagousset, G.; Magnier, E.; Masson, G. Synthesis 2015, 47, 2439-2445.
A mild and simple three-component procedure for the direct vicinal halotrifluoromethylation of styrenes and aliphatic alkenes has been developed in the presence of [Ru(bpy)3](PF6)2 as a photosensitizer and Umemoto’s reagent as the CF3 source. The multicomponent method offers the advantage of short reaction time, moderate to good yields, and mild reaction conditions.
60. « Enamide derivatives: versatile building blocks for total synthesis » Courant, T. ; Dagousset, G. ; Masson, G. Synthesis 2015, 13, 1799-1856.
Enamides and enecarbamates have been shown to be versatile building blocks in organic synthesis. This review describes the development of the enamide chemistry and the utility of the resulting products formed in the synthesis of a variety of important, biologically active molecules.
59. « Formal asymmetric organocatalytic [3 + 2] cyclization between enecarbamates and 3-indolylmethanols – a rapid access to 3-aminocyclopenta[b]indoles » Lebée, C.; Kataja, A.; Blanchard, F.; Masson, G. Chem. Eur. J. 2015, 21, 8399–8402.
A highly enantio- and diastereoselective synthesis of 3-aminocyclopenta[b]indoles has been developed through formal [3+2] cycloaddition reaction of enecarbamates and 3-indolylmethanols. This transformation is catalyzed by a chiral phosphoric acid that achieves simultaneous activation of both partners of the cycloaddition. Mechanistic data are also presented that suggest that the reaction occurs via a stepwise pathway.
58. « Catalytic, highly enantioselective, direct amination of enecarbamates » Dumoulin, A.; Lalli, C.; Retailleau, P.; Masson, G. Chem. Commun. 2015, 51, 5383–5386.
Amination of enecarbamates with dibenzylazodicarboxylate and oxygenated nucleophiles in the presence of a catalytic amount of chiral phosphoric acid afforded optically active stable precursors of α-hydrazinoimines, which were reduced or oxidized, respectively, to vicinal diamines or α-amino acid precursors with excellent yield and enantioselectivity.
57. « Chiral calcium-BINOL phosphate catalyzed diastereo- and enantioselective synthesis of syn-1,2-disubstituted 1,2-diamines: scope and mechanistic studies » Lalli, C.; Dumoulin, A.; Lebée, C.; Drouet, F.; Guérineau, V.; Touboul, D.; Gandon, V.; Zhu, J.; Masson, G. Chem. Eur. J. 2015, 21, 1704–1712.
A highly enantioselective, chiral, Lewis acid calcium–bis(phosphate) complex, Ca[3 a]n, which catalyzes the electrophilic amination of enamides with azodicarboxylate derivatives 2 to provide versatile chiral 1,2-hydrazinoimines 4 is disclosed. The reaction gives an easy entry to optically active syn-1,2-disubstituted 1,2-diamines 6 in high yields with excellent enantioselectivities, after a one-pot reduction of the intermediate 1,2-hydrazinoimines 4. The geometry and nature of the N-substituent of the enamide affect dramatically both the reactivity and the enantioselectivity. Although the calcium–bis(phosphate) complex was a uniquely effective catalyst, the exact nature of the active catalytic species remains unclear. NMR spectroscopy and MS analysis of the various calcium complexes Ca[3]n reveals that the catalysts exist in various oligomer forms. The present mechanistic study, which includes nonlinear effects and kinetic measurements, constitutes a first step in understanding these calcium–bis(phosphate) complex catalysts. DFT calculations were carried out to explore the mechanism and the origin of the enantioselectivity with the Ca[3]n catalysts.
2014
56. « Enamide derivatives: Versatile Building Blocks for Highly Functionalized α,β-Substituted Amines » Bernadat, G.; Masson, G. Synlett 2014, 25, 2842–2867.
As demonstrated by earlier successes with enamines, nitrogen-activated C=C double bonds have considerable potential for use in the construction of various nitrogen-containing products. To expand the applications of this class of substrates, we focused on studying the reactivity of enamides and enecarbamates as promising representatives. Starting from the well-known Povarov reaction, we gradually developed other cycloaddition reactions and, more generally, an extended range of methods for α,β-difunctionalization. Our most recent work, which involves radical processes, has contributed to a significant increase in the diversity of scaffolds accessible from these nitrogenous substrates and is potentially applicable to various natural and bioactive synthetic targets.
55. « Imine and iminium precursors as versatile intermediates in enantioselective organocatalysis » Kataja, A. O.; Masson, G. Tetrahedron 2014, 70, 8783-8815.
54. « One pot and selective intermolecular aryl- and heteroaryltrifluoromethylation of alkenes by photoredox catalysis » Carboni, A.; Dagousset, G.; Magnier, E.; Masson, G. Chem. Commun. 2014, 50, 14197-14200.
We report herein the first photoredox-catalyzed intermolecular aryl- and heteroaryltrifluoromethylation of alkenes. Under the optimized conditions using Umemoto’s reagent as a CF3 source, a wide range of styrenes can be readily difunctionalized, affording the corresponding trifluoromethylated adducts in up to 99% yield.
53. « Photoredox-induced three-component azido- and aminotrifluoromethylation of alkenes » Dagousset, G. Carboni, A.; Magnier, E.; Masson, G. Org. Lett. 2014, 16, 4340-4343.
We report herein a photoredox-catalyzed azidotrifluoromethylation of alkenes. Under the optimized conditions using [Ru(bpy)3(PF6)2] as the photocatalyst and Umemoto’s reagent as the CF3 source, a wide range of substituted styrenes as well as various activated and nonactivated alkenes can readily be difunctionalized, affording β-trifluoromethylated azides or amines in good yields.
52. « Phosphoric Acid Catalyzed Diastereo- and Enantioselective Synthesis of Substituted 1,3- Diaminotetralins » Dagousset, G.; Erb, W.; Zhu, J.; Masson, G. Org. Lett. 2014, 16, 2554-2557.
The reaction of anilines and phenylacetaldehydes in the presence of chiral phosphoric acid afforded optically active 1,2-trans, 2,3-cis 1,3-diaminotetralins in high yields with excellent diastereo- and enantioselectivities. The trans/cis product was readily isomerized to a trans/trans stereoisomer with no significant loss of enantiomeric purity.
51. « Cerium(IV) Ammonium Nitrate Mediated Three-Component alpha-Allylation of Imine Surrogates » Bekkaye, M. ; Masson, G. Org. Lett. 2014, 16, 1510-1513.
A general and practical CAN-mediated oxidative radical α-coupling reaction of various imine surrogates with allylsilanes has been described. This multicomponent process affords β-allylated α-carbamido ethers as stable imine precursors in respectable yields under mild conditions.
50. « Photoredox-Induced Three-Component Oxy-, Amino-, and Carbotrifluoromethylation of Enecarbamates » Carboni, A. ; Dagousset, G. ; Magnier, E. ; Masson, G. Org. Lett. 2014, 16, 1240-1243.
A photoredox-catalzyed trifluoromethylation of enecarbamates process is reported. This pathway uses Togni’s reagent as the CF3 source and follows a radical/cationic pathway. Under the optimized conditions using [Ru(bpy)3(PF6)2] as the photocatalyst, a wide range of substituted enecarbamates can readily be difunctionalized by means of various O, N, and C nucleophiles.
49. « NIS-Assisted Aza-Friedel–Crafts Reaction with alpha-Carbamoysulfides as Precursors of N-Carbamoylimines » George, N. ; Bekkaye, M. ; Alix, A. ; Zhu, J. ; Masson, G. Chem. Eur. J. 2014, 20, 3621-3625.
A general and practical N-iodosuccinimide (NIS)-promoted aza-Friedel–Crafts reaction of various aromatic nucleophiles with N-acylimines generated in situ from α-amidosulfides to give a rapid access to highly functionalized amines is described. The newly developed methodology is very mild, fast, efficient, and complementary.
48. « Chiral Phosphoric Acid-Catalyzed Enantioselective Three-Component Aza-Diels–Alder Reactions of Aminopyrroles and Aminopyrazoles » Brioche,J. ; Courant, T. ; Alcaraz, L. ; Stocks, M. ; Furber, M. ; Zhu, J. ; Masson, G. Adv. Synth. Catal. 2014, 356, 1719-1724.
A highly stereoselective three-component Povarov reaction, catalyzed by (R)- and (S)-BINOL hydrogen phosphate, was achieved for the first time with aminopyrroles and aminopyrazoles as 2-azadiene precursors. A variety of aldehydes, enecarbamates, amino-substituted azines participated in the reaction to afford the tetrahydropyrrolopyridines and tetrahydropyrazolopyridines in good yields with excellent diatereo- and enantioselectivities. A stereochemical model is proposed to account for the observed absolute stereochemistry.
2013
47. « Iron Chloride-Catalyzed Three-Component Domino Sequences : Syntheses of Functionalized alpha-Oxy-N-acylhemiaminals and alpha-Oxyimides » Drouet F. ; Zhu, J. ; Masson, G. Adv. Synth. Catal. 2013, 355, 3563-3569.
The iron(III) chloride-multicatalyzed dioxygenation of enamides with TEMPO in the presence of alcohols has been developed. This multicomponent domino process affords efficient new strategies for the synthesis of α-oxy-N-acylhemiaminals or α-oxyimides in good to excellent yields under mild conditions.
46. « Highly Enantioselective Aza-Diels–Alder Reaction of 1-Azadienes with Enecarbamates Catalyzed by Chiral Phosphoric Acids » He, L. ; Laurent, G. ; Retailleau, P. ; Folléas, B. ; Brayer, J.-L. ; Masson, G. Angew. Chem. Int. Ed. 2013, 52, 11088-11091.
A highly enantio- and diastereoselective synthesis of 6-amino- trisubstituted tetrahydropyridine compounds has been developed through the inverse-electron-demand aza-Diels–Alder reaction of N-aryl α,β-unsaturated ketimines with enecarbamates (E)-1. Chiral phosphoric acid catalysts achieve simultaneous activation of both the 1-azadiene and dienophile partners.
45. « Ugi Four-Component Reaction of Alcohols : Stoichiometric and Catalytic Oxidation/MCR Sequences » Drouet, F. ; Masson, G. ; Zhu, J. Org. Lett. 2013, 15, 2854-2857.
A new, simple, and efficient procedure for the one-pot Ugi four-component reaction of alcohols instead of aldehydes is described. Using a stoichiometric amount of IBX or only 1–2% of sodium 2-iodobenzenesulfonate in the presence of Oxone, a wide range of primary alcohols were oxidized to the aldehyde that were directly engaged in the Ugi four-component reaction to afford α-acetamidoamides in good to excellent yields.
44. « Metal-Free Dioxygenation of Enecarbamates Mediated by a Hypervalent Iodine Reagent » Bekkaye, M. ; Su, Y. ; Masson, G. Eur. J. Org. Chem. 2013, 19, 3978-3982.
A general method for the vicinal dioxygenation of enecarbamates was developed by using PhI(OAc)2 as the sole oxidant under extremely mild conditions, thus avoiding starting material decomposition. This methodology, which is an alternative to common dioxygenation processes catalyzed by transition metals, provides easy access to functionalized β-acetoxy-α-amido ethers and α-amido-β-oxytetrahydrofurans in moderate to good yields.
43. « Chiral phosphoric Acid-Catalyzed Enantioselective Aza-Friedel-Crafts Alkylation of Indoles with gamma-Hydroxy-gamma-lactams » Courant, T. ; Kumarn, S. ; He, L. ; Retailleau, P. ; Masson, G. Adv. Synth. Catal. 2013, 355, 836-840.
An enantioselective aza-Friedel–Crafts reaction of indoles with γ-hydroxy-γ-lactams using a chiral phosphoric acid catalyst is reported. The approach described herein provides an efficient access to 5-indolylpyrrolidinones in good to quantitative yields and excellent enantioselectivities (up to >99% ee). The results suggest that the reaction may proceed via N-acyliminium intermediates associated with the chiral phosphoric acid anion.
42. « Catalytic Enantioselective [4+2]-Cycloaddition : a Strategy to Access Aza-Hexacycles » Masson, G. ; Lalli, C. ; Benohoud, M. ; Dagousset, G. Chem. Soc. Rev. 2013, 42, 902-923.
The aza-Diels Alder reaction has become one of the most widely used synthetic tools for the preparation of N-containing 6-membered heterocycles. Numerous important developments have been reported to render this reaction catalytic and enantioselective. This tutorial review highlights strategies and recent advances to achieve high efficiency and selectivity through the use of organocatalysts and transition metal complexes, allowing also the extension of this transformation substrate scope.
2012
41. « Chiral Phosphoric Acid Catalyzed Inverse-Electron-Demand Aza-Diels–Alder Reaction of Isoeugenol Derivatives » He, L. ; Bekkaye, M. ; Retailleau, P. ; Masson, G. Org. Lett. 2012, 14, 3158-3161.
Highly enantio- and diastereoselective three-component inverse electron-demand aza-Diels–Alder reaction of aldehydes, anilines, and isoeugenol derivatives catalyzed by a chiral phosphoric acid catalyst are reported. A wide variety of 2,3,4-trisubstituted tetrahydroquinolines containing an aryl group at the 4-position were obtained in a one-pot process with good to high yields and excellent stereoselectivities (>95:5 dr and up to >99% ee).
40. « Amidation of Aldehydes and Alcohols via a-Iminonitriles and a Sequential Oxidative Three component Strecker Reaction/Thio-Michael Addition/Alumina-promoted Hydrolysis to Access a-Mercaptoamides from Aldehydes, Amines and Thiols » Gualtierotti, J-B. ; Schumacher, X. ; Fontaine, P. ; Masson, G. ; Wang, Q. ; Zhu, J. Chem. Eur. J. 2012, 18, 14812-14819.
Mild and general alumina-promoted hydrolysis conditions for converting α-iminonitriles into carboxamides have been developed. In combination with the oxidative three-component Strecker reaction, the one-pot direct amidation of aldehydes and alcohols is reported. Subsequently, an Yb(OTf)3-catalyzed Michael addition of thiols to α,β-unsaturated α-iminonitriles is reported for the synthesis of β-mercapto-α-iminonitriles. The successful integration of an oxidative Strecker reaction, thio-Michael addition, and neutral-alumina-promoted hydrolysis of β-mercapto-α-iminonitriles into a three-component one-pot process allowed us to develop the direct conversion of amines, aldehydes, and thiols into β-mercaptoamides. All of these procedures were applicable to aromatic and aliphatic amines and aldehydes.
39. « Organocatalytic Enantioselective One-pot Four-component Ugi-type Multicomponent Reaction for the Synthesis of Epoxy-tetrahydropyrrolo[3,4-b]pyridin-5-ones » Su, Y. ; Bouma, M. J.; Alcarez, L. ; Stocks, M. ; Furber, M. ; Masson, G. ; Zhu, J. Chem. Eur. J. 2012, 18, 12624-12627.
In the presence of a catalytic amount of chiral BINOL-derived phosphoric acid (TRIP), the reaction of an α-isocyanoacetate 1, an aldehyde 2, and an aniline 3, followed by addition of a toluene solution of α,β-unsaturated acyl chloride 4 afforded the oxa-bridged tricycle 5 in excellent yield, diastereoselectivity, and enantioselectivity. Six chemical bonds, five stereogenic centers, and three cycles were formed in this one-pot four-component reaction.
38. « Highly enantioselective electrophilic -bromination of enecarbamates : chiral phosphoric acid and calcium phosphate salt catalysts » Alix, A. ; Lalli, C. ; Retailleau, P. ; Masson, G. J. Am. Chem. Soc. 2012, 134, 10389-10392.
Metal-free chiral phosphoric acids and chiral calcium phosphates both catalyze highly enantio- and diastereoselective electrophilic α-bromination of enecarbamates to provide an atom-economical synthesis of enantioenriched vicinal haloamines. Either enantiomer can be formed in good yield with excellent diastereo- and enantioselectivity simply by switching the catalyst from a phosphoric acid to its calcium salt.
37. « Chiral Phosphoric Acid-Catalyzed Enantioselective Three-Component Povarov Reaction Using Cyclic Enethioureas as Dienophiles : Stereocontrolled Access to Enantioenriched Hexahydropyrroloquinolines » Dagousset, G. ; Retailleau, P. ; Masson, G. ; Zhu, J. Chem. Eur. J. 2012, 18, 5869-5873.
Phosphoric acid-catalyzed enantioselective three-component Povarov reactions of aldehydes, anilines, and endocyclic enethioureas have been developed (see scheme). This process afforded hexahydropyrroloquinolines in high yields with excellent diastereo- and enantioselectivities. The presence of the thiourea functionality is crucial for the enantioselectivity of the reaction.
36. « Exploiting the Divergent Reactivity of Isocyanoacetates : One-Pot Three-Component Synthesis of Functionalized Angular Furoquinolines » Bouma, M. J. ; Bonne, D. ; Masson, G. ; Zhu, J. Eur. J. Org. Chem. 2012, 3, 475-479.
A one-pot, three-component synthesis of polysubstituted furo[2,3-c]quinoline 1 was developed based on the new reactivity profile of α-(4-nitrophenyl)-α-isocyanoacetates. Simply mixing aldehydes 2, ortho-alkynylanilines 3, and α-(4-nitrophenyl)-α-isocyanoacetates 4 in methanol at room temperature, followed by addition of toluene and heating to reflux, provided the polysubstituted furo[2,3-c]quinolines in moderate to excellent yields. Mechanistically, the three-component reaction of 2, 3, and 4 leading to 5-alkoxyoxazoles was followed by a domino sequence involving Diels–Alder/retro-Diels–Alder/oxidation reactions. In this one-pot process, five chemical bonds were created with concurrent formation of two heterocyclic rings. The reaction was performed under thermal conditions and no external reagent was needed to promote this mechanistically intriguing reaction.
35. « Photoredox Initiated α-Alkylation of Imines via a Three-Component Radical/Cationic Reaction » Courant, T. Masson, G. Chem. Eur. J. 2012, 18, 423-427.
The photoredox-mediated alkylation of enamides with diethyl bromomalonate in the presence of alcohols has been developed. This multicomponent domino process affords β-alkylated α-carbamido ethers as stables imine surrogates in good to excellent yields under mild conditions.
2011
34. « Catalytic Enantioselective Cycloaddition with Chiral Lewis Bases » Lalli, C. ; Brioche, J. ; Bernadat, G. ; Masson, G. Curr. Org. Chem. 2011, 15, 4108-4127.
Over the past decade, chiral Lewis base-catalysed cycloadditions has brought about numerous methods for the enantioselective synthesis of carbocycles and heterocycles. This short review covers some of the advances made in the area of absolute stereocontrol in this higher order cycloaddition.
33. « Chiral Phosphoric Acid-Catalyzed Enantioselective Three-Component Povarov Reaction Using Enecarbamates as Dienophiles: Highly Diastereo- and Enantioselective Synthesis of Substituted 4-Aminotetrahydroquinolines » Dagousset, G. ; Zhu, J. ; Masson, G. J. Am. Chem. Soc. 2011, 133, 14804-14813.
A chiral phosphoric acid (5)-catalyzed three-component Povarov reaction of aldehydes 2, anilines 3, and enecarbamates 4 afforded cis-4-amino-2-aryl(alkyl)-1,2,3,4-tetrahydroquinolines 1 in high yields with excellent diastereoselectivities (>95%) and almost complete enantioselectivities (up to >99% ee). The reaction was applicable to a wide range of anilines bearing electron-donating (OMe) and electron-withdrawing groups (e.g., Cl, CF3, NO2) and allowed, for the first time, aliphatic aldehydes to be employed in the enantioselective Povarov reaction. With β-substituted acyclic enecarbamates, 2,3,4-trisubstituted 1,2,3,4-tetrahydroquinolines with three contiguous stereogenic centers were produced in excellent diastereo- and enantioselectivities (87 to >99% ee). A detailed study of the active catalytic species allowed us to reduce the catalyst loading from 10% to 0.5% with no deterioration of enantiomeric excess. In addition, mechanistic studies allowed us to conclude unequivocally that the Povarov reaction involving enecarbamate as dienophile proceeded via a stepwise mechanism. The key role of the free NH function of the enecarbamate in the success of this transformation was demonstrated. NMR experiments indicating the catalyst–substrate interaction as well as a linear correlation between catalyst and product ee’s were also documented.
32. « A practical, one-pot multicomponent synthesis of α-amidosulfides and its application as latent N-acylimines in Friedel-Crafts reaction » George, N. ; Bekkaye, M. ; Masson, G. ; Zhu, J. Eur. J. Org. Chem. 2011, 20-21,3695-3699. Special issue dedicated to Women in Chemistry.
A novel one-pot, three-component synthesis of N-acyl or N-carbamoyl-α-amidosulfides 4 is described. The three-component reaction of aldehydes 1, primary carbamates (or amides) 2 and phenylsulfinic acid (6a) afforded α-amidosulfones 7, which after addition of sodium thiolate were in situ transformed into stable α-amidosulfides 4 in good to excellent yields. We demonstrated that silver salts or Brønsted acids were able to promote the formation of aliphatic and aromatic N-acylimines from 4 in quantitative yield under mild conditions. The phosphoric acid catalyzed Friedel–Crafts alkylation of 3-substituted indoles with α-amidosulfides 4 leading to 2,3-disubstitued indoles was also documented.
31. « Cinchona Alkaloid-Amides catalyzed Enantioselective Formal [2+2] Cycloadditions of Allenoates and imines : Enantioselective Synthesis of 2,4-Substituted Azetidines » Denis, J. B. ; Masson, G. ; Retailleau, P. ; Zhu, J. Angew. Chem. Int. Ed. 2011, 50, 5356-5360. Selected by Synfacts, 2011, 792.
The quinidine amide 1 catalyzed [2+2] cycloaddition between N-sulfonylimines 2 and alkyl 2,3-butadienoates 3 afforded the R-configured azetidines 4 in excellent yields and enantioselectivities (M.S.=molecular sieve). The S enantiomer was obtained when a quinine amide catalyst, the pseudoenantiomer of 1, was used.
30. « Asymmetric Synthesis 2,4,6-trideoxy-4-(dimethylamino)-3-C-methyl-L-lyxohexopyra-nose (Lemonose) » Bernadat, G. ; George, N. ; Couturier, C. ; Masson, G. ; Schlama, T. ; Zhu, J. Synlett, 2011, 576-578.
L-Lemonose, the glycosidic part of (-)-lemonomycin, has been synthesized in ten steps with 18% overall yield from D-threonine. The key steps are a double, highly diastereoselective Grignard addition to a Weinreb amide and a chemoselective oxidation of a primary alcohol in the presence of a secondary alcohol, a tertiary alcohol and a tertiary amine, leading directly to the lactol.
29. « Chiral Calcium Organophosphate-Catalyzed Enantioselective Electrophilic Amination of Enamides » Drouet, F. ; Lalli, C. ; Liu, H. ; Masson, G. ; Zhu, J. Org. Lett. 2011, 13, 94-97.
Highly enantioselective direct amination of enamides catalyzed by chiral nonracemic calcium bis(phosphate) complex 3g afforded optically active 1,2-hydrazinoimines 4. Following a subsequent in situ hydrolysis or reduction, 2-hydrazinoketones 5 or syn-1,2-disubstituted 1,2-diamines 6 were obtained in high yields and excellent enantiomeric excess.
28. « Exploiting the Divergent Reactivity α-Isocyanoacetate : Multicomponent Synthesis of 5-Alkoxyoxazole and Heterocycles » Lalli, C. ; Bouma, M. J. ; Bonne, D. ; Masson, G. ; Zhu, J. Chem. Eur. J. 2011, 17, 880-889.
A novel multicomponent synthesis of 5-alkoxyoxazoles, based on a new reactivity profile of α-isocyanoacetates, is described. Thus, simply heating a solution of amine, aldehyde, and α-(EWG-phenyl)-α-isocyanoacetate or α-(4-pyridyl)-α-isocyanoacetate (EWG=electron-withdrawing group) in toluene provided 5-alkoxyoxazoles in good to excellent yields. Reaction of the 5-alkoxyoxazoles with various α,β-unsaturated acyl chlorides led to the formation of epoxytetrahydropyrrolo[3,4-b]pyridin-5-ones by a domino N-acylation/Diels–Alder cycloaddition sequence. Subsequent fragmentation under basic conditions provided 6,7-dihydro-5H-pyrrolo[3,4-b]pyridin-5-ones. A four-component synthesis of the pyridin-5-one compounds, without isolation of the 5-alkoxyoxazole, was subsequently developed.
2010
27. « SmI2-Mediated Reductive Cross-Coupling Reactions of α-Cyclopropyl Nitrones » Burchak, O. ; Masson, G. ; Py, S. Synlett 2010, 1623-1626.
Three new α-cyclopropyl nitrones have been synthesized as mechanistic probes for reductive cross-coupling reactions of nitrones. The α-cyclopropylcarbinyl radical intermediate formed by single electron transfer from SmI2 to these nitrones is not prone to ring opening, due to a unique stabilization by the vicinal N-O-Sm system. Consequently, β-cyclopropyl hydroxylamines could be prepared in high yield from α-cyclopropyl nitrones.
26. « Zinc Chloride Promoted Formal Oxidative Coupling of Aromatic AldehydelIsocyanides to α-Ketoamides » Bouma, M. ; Masson, G. ; Zhu, J. J. Org. Chem. 2010, 75, 2748-2751.
Reaction of aromatic aldehydes and isocyanides in the presence of N-methylhydroxyamine, acetic acid, and zinc chloride affords the aryl α-ketoamides in moderate to good yields.
25. « Passerini three-component Reaction of Alcohols under Catalytic Aerobic Oxidative Conditions » Brioche, J. ; Masson, G. ; Zhu, J. Org. Lett. 2010, 12, 1432-1435.
Alcohols instead of aldehydes were used in the Passerini three-component reaction under catalytic aerobic conditions. Mixing alcohols, isocyanides, and carboxylic acids in toluene in the presence of a catalytic amount of cupric chloride, NaNO2, and TEMPO afforded, under an oxygen atmosphere, the P-3CR adducts in good yields.
24. « Enantioselective Aza-Morita-Baylis-Hillman Reaction Using Aliphatic α-Amidosulfones as Imine Surrogates » Abermil, N. ; Masson, G. ; Zhu, J. Adv. Synth. Catal. 2010, 352, 656-660. Selected by Synfacts, 2010, 595.
The bifunctional catalyst 6′-deoxy-6′-acylamino-β-isocupreidine (1) served both as a base to trigger the in situ generation of N-sulfonylimine from readily available α-amidosulfones and as a chiral nucleophile to initiate the enantioselective aza-Morita–Baylis–Hillman (aza-MBH) reaction. α-Methylene-β-amino-β-alkyl carbonyl compounds, difficultly accessible previously, can now be synthesized in excellent yields and enantioselectivities.
2009
23. « Chiral Brønsted Acid-Catalyzed Enantioselective Multicomponent Mannich Reaction : Synthesis of anti-1,3-Diamines Using Enecarbamates as Nucleophiles » Dagousset, G. ; Drouet, F. ; Masson, G. ; Zhu, J. Org. Lett. 2009, 11, 5546-5549.
Reaction of aldehydes 2, anilines 3, and enecarbamates 4 in dichloromethane in the presence of EtOH and a catalytic amount of chiral phosphoric acid 5 afforded the Mannich adducts which were in situ reduced to anti-1,2-disubstituted 1,3-diamines 1 in excellent diastereoselectivity and enantioselectivity.
22. « Invertible Enantioselectivity in 6’-Deoxy-6’-acylamino-β-isocupreidine-Catalysed Asymmetric Aza-Morita-Baylis-Hillman Reaction : Key role of a Achiral Additive » Abermil, N. ; Masson, G. ; Zhu, J. Org. Lett. 2009, 11, 4648-4651.
The β-ICD (1a) or β-ICD-amide (1e)-catalyzed aza-Morita−Baylis−Hillman reaction between N-sulfonylimines 3 and alkyl vinyl ketones 4 produced the (R)-enriched adducts 5. By adding a catalytic amount of β-naphthol (2a), the enantioselectivity of the same reaction was inversed leading to (S)-5 in excellent yields and enantioselectivities. Both aromatic and aliphatic imines are accepted as substrates for this reaction.
21. « Catalytic Asymmetric Passerini Type Reaction : Chiral Aluminium–Organophosphate-Catalyzed Enantioselective α-Addition of Isocyanides to Aldehydes » Yue, T. ; Wang, M.-X. ; Wang, D.-X., Masson, G. ; Zhu, J. J. Org. Chem. 2009, 74, 8396-8399.
A chiral Lewis acid catalyst was prepared by mixing 2 equiv of chiral binol-derived organophosphoric acid and 1 equiv of Et2AlCl. In the presence of a catalytic amount of [4j]2Al(III)Cl complex (0.05 equiv), reaction between α-isocyanoacetamides (2) and aldehydes (3) afforded the corresponding 5-aminooxazoles (1) in good yields and enantioselectivities. Complex [4j]2Al(III)Cl isolated as a white solid displayed similar reactivity as that prepared in situ.
20. « Brønsted Acid Catalyzed Enantioselective Three-Component Reaction Involving the α-Addition of Isocyanides to Imines » Yue, T. ; Wang, M.-X. ; Wang, D.-X., Masson, G. ; Zhu, J. Angew. Chem. Int. Ed. 2009, 48, 6717-6721.
Three-component reactions of aldehydes, anilines, and α-isocyanoacetamides in the presence of a catalytic amount of chiral phosphoric acid afforded the 5-(1-aminoalkyl)-5-aminooxazole 1 in excellent yields and moderate to good enantiomeric excess (see scheme).
19. « Chiral Brønsted Acid-Catalyzed Enantioselective Three-Component Povarov Reaction » Liu, H. ; Dagousset, G. ; Masson, G. ; Retailleau, P. ; Zhu, J. J. Am. Chem. Soc. 2009, 131, 4598-4599. Selected by Synfacts, 2009, 681. Selected by JACS Select.
The three-component Povarov reaction of aldehydes (2), anilines (3), and benzyl N-vinylcarbamate 4 in the presence of 0.1 equiv of chiral phosphoric acid 5 afforded cis-2,4-disubstituted tetrahydroquinolines (1) in good yields and excellent enantiomeric excesses. The shortest synthesis of torcetrapib reported to date, which features this key three-component reaction, is documented.
18. « Synthesis of Pyrroles by Consecutive Multicomponent Reaction/[4+1] Cycloaddition of α-Iminonitriles with Isocyanides » Fontaine, P. ; Masson, G. ; Zhu, J. Org. Lett. 2009, 11, 1555-1558. Selected by Synfacts, 2009, 599.
[4 + 1] Cycloaddition of α,β-unsaturated imidoyl cyanide (2-cyano-1-azadienes) with isocyanides in the presence of a catalytic amount of AlCl3 afforded polysubstituted 2-amino-5-cyanopyrroles in good to excellent yields. In combination with the IBX/TBAB-mediated oxidative Strecker reaction, this important heterocycle is readily synthesized in two steps from simple starting materials.
17. « IBX/TBAB-mediated oxidation of primary amines to nitriles » Drouet, F. ; Fontaine, P. ; Masson, G. ; Zhu, J. Synthesis, 2009, 1370-1374.
The combination of o-iodoxybenzoic acid (IBX) and tetrabutylammonium bromide (TBAB) efficiently oxidizes primary amines to the corresponding nitriles in good to excellent yield under mild conditions. The reaction is racemization-free when applied to a chiral lysine derivative.
16. « Synthetic Studies on (−)-Lemonomycin : An Efficient Asymmetric Synthesis of Lemonomycinone Amide » Wu, Y.-C. ; Bernadat, G. ; Masson, G. ; Couturier, C. ; Schlama, T. ; Zhu, J. J. Org. Chem. 2009, 74, 2046-2052. Selected by Synfacts, 2009, 1070.
Asymmetric synthesis of lemonomycinone amide (2) was accomplished from readily accessible starting materials. Enantioselective alkylation of N-(diphenylmethylene)glycine tert-butyl ester (11) by 5-tert-butyldimethylsilyloxy-2,4-dimethoxy-3-methylbenzyl bromide (10) in the presence of Corey-Lygo’s phase transfer catalyst [O-(9)-ally-N-(9′-anthracenylmethyl) cinchonidium bromide, 0.1 equiv] afforded, after chemoselective hydrolysis of the imine function (THF/H2O/AcOH), the substituted L–tert-butyl phenylalanate 13 in 85% yield. A Pictet−Spengler reaction of 14 with benzyloxyacetaldehyde (15) provided the 1,3-cis-disubstituted tetrahydroisoquinoline 16 in 85% yield as a single diastereomer. Coupling of hindered secondary amine 16 with amino acid 9 was accomplished under carefully controlled conditions to furnish the amide 22, which was in turn converted to hemiaminal 24. A hafnium triflate catalyzed conversion of hemiaminal to α-amino thioether followed by a silver tetrafluoroborate promoted intramolecular Mannich reaction of 26 afforded the tetracycle 27 in excellent overall yields. Debenzylation of 27 [Pd(OH)2, H2, MeOH, 0 °C], removal of N-Boc function (aqueous 3 N HCl, MeOH/H2O), and oxidation of hydroquinone to quinone [(NH4)2Ce(NO3)6, H2O, rt] afforded the lemonomycinone amide 2 in 76% yield over three steps.
2008
15. « Highly Enantioselective Aza-Morita−Baylis−Hillman Reaction Catalyzed by Bifunctional β-Isocupreidine Derivatives » Abermil, N. ; Masson, G. ; Zhu, J. J. Am. Chem. Soc. 2008, 130, 12596-12597. Selected by Synfacts, 2008, 1215.
The aza-MBH reaction of imines 1 and β-naphthyl acrylate 2 in the presence of C-6′ modified β-isocupreidine derivative 1c (0.1 equiv) and β-naphthol 5 (0.1 equiv) afforded the corresponding (3S)-aza-MBH adducts 4 in high yield and excellent enantiomeric excess. These catalytic conditions allowed the aliphatic imines to be employed for the first time as electrophilic partners of the aza-MBH reaction. The coexistence of two H-bond donors with different acidic strengths was found to be crucial for the observed high enantioselectivity.
14. « One-pot three-component synthesis of α- iminonitriles by IBX/TBAB-mediated oxidative Strecker reaction » Fontaine, P. ; Chiaroni, A. ; Masson, G. ; Zhu, J. Org. Lett. 2008, 10, 1509-1512.
The reaction of aldehydes, amines, and TMSCN in the presence of 2-iodoxybenzoic acid (IBX) and tetrabutylammonium bromide (TBAB) afforded α-iminonitriles in good to excellent yields under mild conditions. The presence of TBAB is essential for this transformation. The methodology was applied to a two-step synthesis of indolizidine via a microwave-assisted intramolecular cycloaddition of α-iminonitrile.
13. « Synthesis of α-ketoamide via Molecular Sieves-Promoted Formal Oxidative Coupling of Aliphatic Aldehyde and Isocyanide » Grassot, J.- M. ; Masson, G. ; Zhu, J. Angew. Chem. Int. Ed. 2008, 46, 947-950. Selected as “Hot Paper”
The reaction of an aldehyde, an isocyanide, N-methylhydroxylamine, and acetic acid in the presence of 4 Å molecular sieves afforded α-ketoamides in moderate to good yields (see scheme). In contrast, 3 Å molecular sieves did not promote the desired multicomponent reaction. R1=alkyl; R2=alkyl, aryl.
2007
12. « Ammonium Chloride Promoted Three-Component Synthesis of 5-Iminooxazoline and Its Subsequent Transformation to Macrocyclodepsipeptide » Pirali, T. ; Tron, G. C. ; Masson, G. ; Zhu, J. Org. Lett. 2007, 9, 5275-5278.
A three-component reaction of an α,α-disubstituted α-isocyanoacetamide, an aldehyde and an amino alcohol afforded the 5-iminooxazoline, which, upon saponification, cyclized under acidic conditions to provide the macrocyclodepsipeptide in good overall yield.
11. « The Enantioselective Morita-Baylis-Hillman Reaction and Its Aza Counterpart » Masson, G. ; Housseman, C. ; Zhu, J. Angew. Chem. Int. Ed. 2007, 46, 4614-4628.
The development of asymmetric Morita–Baylis–Hillman (MBH) reactions has evolved dramatically over the past few years, parallel to the emerging concept of bifunctional organocatalysis. Whereas organocatalysis is starting to compete with metal-based catalysis in several important organic transformations, the MBH reaction belongs to a group of prototypical reactions in which organocatalysts already display superiority over their metal-based counterparts. This Minireview summarizes recent mechanistic insights and advances in the design and synthesis of small organic molecules for enantioselective MBH and aza-MBH reactions.
10. « Rapid Synthesis of Cyclodepsipeptides Containing a Sugar Amino Acid or a Sugar Amino Alcohol by a Sequence of a Multicomponent Reaction and Acid-Mediated Macrocyclization » Bughin, C. ; Masson, G. ; Zhu, J. J. Org. Chem. 2007, 72, 1826-1829.
Cyclodepsipeptides incorporating a sugar amino acid (alcohol) have been synthesized. A three-component reaction of a sugar amino acid (SAA) derivative, an aldehyde, and a dipeptide isonitrile in refluxing methanol afforded the corresponding 5-aminooxazole which, after saponification, underwent a trifluoroacetic acid promoted macrocyclization to furnish the cyclic sugar amino acids.
2006
9. « Intramolecular Staudinger Ligation towards Biaryl-Containing Lactams » Masson, G. ; Hartog, T. ; Schoemaker, H. E. ; Hiemstra, H. ; van Maarseveen, J. H. Synlett, 2006, 865-868.
Both 15- and 16-membered biaryl-type lactams were prepared in good yield using the intramolecular Staudinger ligation strategy.
2005
8. « Mild and Chemoselective Peptide-Bond Cleavage of Peptides and Proteins at Azido Homoalanine » Back, J. W. ; David, O. ; Kramer, G. ; Masson, G. ; Kasper, P. T. ; Koning, L. J. ; Jong, L., van Maarseveen J. H. ; Koster, C. G. Angew. Chem. Int. Ed. 2005, 44, 7946-7950.
Cleavage of peptides containing the non-natural but proteogenic amino acid azido homoalanine can be accomplished by phosphine- or dithiol-mediated reductive activation of the azide functionality in aqueous buffers (see scheme). The resulting C-terminal lactone of one peptide fragment allows facile further derivatization.
7. « Cis-Stereoselective SmI2-promoted reductive coupling of keto-nitrones : first synthesis of 1-epitrehazolamine » Masson, G. ; Philouze, C. ; Py, S. Org. Biomol. Chem. 2005, 2067-2069.
An expeditious synthesis of 1-epitrehazolamine is presented from readily available 2,3,4,6-tetra-O-benzyl-D-glucose. The key step involves a samarium diiodide-promoted reductive cyclization of a masked keto-nitrone to form a five-membered ring aminocyclitol. The excellent cis selectivity observed in this nitrone–ketone reductive coupling contrasts surprisingly with the trans selectivity of ketone–oxime reductive couplings.
2003
6. « A Concise Formal Synthesis of (S)-Vigabatrin Based on Nitrone Umpolung » Masson, G. ; Zeghida W. ; Cividino, P. ; Py, S. ; Vallée, Y. Synlett 2003, 1527-1529.
A short, formal synthesis of (S)-vigabatrin is described, from readily available 2,3-diprotected d-glyceraldehyde (5). The key step of this synthesis involves a samarium diiodide-induced reductive coupling of the corresponding nitrone with alkyl acrylates.
5. « SmI2-Induced Umpolung of the C=N Bond : First Reductive Conjugate Addition of Nitrones to alpha,beta-Unsaturated Esters » Masson, G. ; Cividino, P. ; Py, S. ; Vallée, Y. Angew. Chem. Int. Ed. 2003, 42, 2265-2268.
Umpolung of the C=N bond of nitrones by reduction with SmI2, generates a species that adds smoothly to α,β-unsaturated esters. The corresponding γ-N-hydroxyamino esters are obtained with excellent stereoselection.
2002
4. « A General for the Practical Synthesis of Nojirimycin C-Glycosides and Analogs. Extension to the First Reported Example of an imino Sugar 1-Phosphonate » Godin, G. ; Compain, P. ; Masson, G. ; Martin, O. J. Org. Chem. 2002, 67, 6960-6970.
An efficient and versatile strategy for the synthesis of nojirimycin C-glycosides and related compounds with full stereocontrol is reported. The key steps of the process are the addition of organometallic reagents onto an L-sorbose-derived imine (13) followed by an internal reductive amination. The addition step, which controls the α- vs β-configuration at the pseudoanomeric center in the final product, is highly diastereoselective (re-face addition), and the stereoselectivity can be effectively inverted by adding an external monodentate Lewis acid (si-face addition). The complete synthesis could be achieved in 10 steps only from commercially available 2,3;4,6-di-O-isopropylidene-α-L-sorbofuranose and provided α- or β-1-C-substituted 1-deoxynojirimycin derivatives in 27−52% overall yield. The strategy was successfully extended to the first example of an iminosugar 1-phosphonate. The methodology provides access to a wide range of biologically relevant glycoconjugate mimetics in which the glycosidic function is replaced by an imino-C-glycosidic linkage.
3. « Samarium Diiodide-Induced Reductive Cross-Coupling of Nitrones with Aldehydes and Ketones » Masson, G. ; Py, S. ; Vallée, Y. Angew. Chem. Int. Ed. 2002, 41, 1772-1775.
Highly substituted amino alcohols or N-hydroxyamino alcohols can be prepared in good yield through a novel reductive cross-coupling of nitrones with aldehydes or ketones (see scheme). The reactions are fast and highly chemoselective, that is, no homocoupling or reduction products are formed.
2001
2. « One-pot synthesis of functionalized nitrones from nitro compounds » Gautheron-Chapoulaud, V. ; Pandya, S. U. ; Cividino, P. ; Masson, G. ; Py, Sandrine ; Vallée, Y. Synlett 2001, 1281-1283.
The zinc-mediated reduction of nitroalkanes and nitroarenes in the presence of aldehydes is an efficient method to synthesize a wide range of nitrones. This method is mild enough to accommodate a variety of functional groups. It is particularly useful when the intermediate hydroxylamines are unstable and/or water-soluble. We used it to prepare several aromatic, aliphatic and highly functionalized sugar-derived nitrones.
2000
1. « A New, Stereocontrolled Approach to Imino Sugar C-Glycosides from L-Sorbose » Masson, G. ; Compain, P. ; Martin, O. R. Org. Lett. 2000, 19, 2971-2974.
The efficient synthesis of the iminoalditols derivatives 1 and 2 (nojirimycin α-C-glycosides) has been achieved in 10 steps from commercially available 2,3;4,6-di-O-isopropylidene-α-L-sorbofuranose in an overall yield of 23−27%.
Book chapters
« Synthesis [of 1-Bromo-n-Heteroatom-Functionalized Alkanes (n ≥ 2) with both functions formed simultaneously] by Addition across C=C Bonds » Dagousset, G., Masson, G. Thieme Chemistry – Science of Synthesis 2014.
« Asymmetric Morita-Baylis-Hillman Reaction and its Aza Analogue » Masson, G., Zhu, J. Stereoselective Synthesis: Stereoselective Reactions of Carbonyl and Imino Groups, Thieme Chemistry – Science of Synthesis 2011, Vol. 2], 735-784.
« Multicomponent Syntheses of Macrocycles » Masson, G., Neuville, L.; Bughin, C.; Fayol, A. Zhu, J. Synthesis of Heterocycles via Multicomponent Reactions II, Topics in Heterocyclic Chemistry 2010, 25, 1-24.
« Development of multicomponent and domino reactions based on α-isocyanoacetamide and 5-aminooxazole » Masson, G., Neuville, L.; Zhu, J. in Seminars in Organic Synthesis, XXXIII A Corbella Summer School, Italian Chemical Society 2008, 11-36.


