Notre thématique s’appuie sur des approches intégrées pour l’étude et la valorisation des métabolites secondaires isolés de plantes et de leurs micro-organismes associés. Nous possédons des compétences transverses allant de l’isolement et l’identification de produits naturels à la synthèse organique avec quatre objectifs complémentaires et interdépendants :
- Étudier la diversité chimique des plantes tropicales
- Découvrir de nouvelles molécules naturelles bioactives
- Améliorer leur activité par la mise en œuvre de projets en chimie médicinale
- Explorer leur mécanisme d’action
Principales thématiques de recherche développées actuellement au laboratoire :
Composés à visée antitumorale
Composés agissant sur des protéines de l’apoptose
A. Abou Samra, A. Robert, C. Gov, L. Favre, L. Eloy, E. Jacquet, J. Bignon, J. Wiels, S. Desrat, F. Roussi. J. Med. Chem. 2018, 148, 26-38.
Dual inhibitors of the pro-survival proteins Bcl-2 and Mcl-1 derived from natural compound meiogynin A. Thirty analogues of natural meiogynin A, a pan-Bcl-2 inhibitor, were prepared in order to elaborate cytotoxic compounds on specific cancer cells overexpressing one or more proteins of the Bcl-2 family. The interaction of all the new analogues with Bcl-xL, Mcl-1 and Bcl-2 proteins was first evaluated by fluorescence polarization assay (FPA) and showed that modulation of the lateral chain has a dramatic impact as subtle changes significantly modify the activity on the target proteins. The acetoxymethyl prodrugs of the two most active compounds were then elaborated to determine their cytotoxicity on B cell lines. A strong cytotoxic effect on BL2, RS4 ;11 and H929 cells was observed with a triazole prodrug that induces apoptosis.
DOI : 10.1016/j.ejmech.2018.01.100

C. Geny, A. Abou Samra, P. Retailleau, B. I. Iorga, H. Nedev, K. Awang, F. Roussi, M. Litaudon, V. Dumontet. J. Nat. Prod. 2017, 80, 3179-3185.
(+)- and (−)-Ecarlottones, Uncommon Chalconoids from Fissistigma latifolium with Pro-Apoptotic Activity. Four new compounds, (+)- and (−)-ecarlottone (1), (±)-fislatifolione (5), (±)-isofislatifolione (6), and (±)-fislatifolic acid (7), and the known desmethoxyyangonin (2), didymocarpin-A (3), and dehydrodidymocarpin-A (4) were isolated from the stem bark of Fissistigma latifolium, by means of bioassay-guided purification using an in vitro affinity displacement assay based on the modulation of Bcl-xL/Bak and Mcl-1/Bid interactions. The structures of the new compounds were elucidated by NMR spectroscopic data analysis, and the absolute configurations of compounds (+)-1 and (−)-1 were assigned by comparison of experimental and computed ECD spectra. (−)-Ecarlottone1 exhibited a potent antagonistic activity on both protein–protein associations with Ki values of 4.8 μM for Bcl-xL/Bak and 2.4 μM for Mcl-1/Bid.
DOI : 10.1021/acs.jnatprod.7b00494

S. Desrat, C. Remeur, F. Roussi. Org. Biomol. Chem. 2015, 13, 5520-5531.
Development of an efficient route toward meiogynin A-inspired dual inhibitors of Bcl-xL and Mcl-1 anti-apoptotic proteins. The synthesis, on a large scale, with very good yield and er via an efficient strategy, of a chiral 4-substituted 2-cyclohexenone intermediate, was a milestone in the synthesis of seven analogues of meiogynin A, a natural sesquiterpenoid dimer.

S. Desrat, C. Remeur, C. Geny, G. Rivière, C. Colas, V. Dumontet, N. Birlirakis, B. Iorga, F. Roussi. Chem. Comm. 2014, 50, 8593-8596.
From meiogynin A to the synthesis of dual inhibitors of Bcl-xL and Mcl-1 anti-apoptotic proteins. The synthesis of one of the most potent dual inhibitor of the anti-apoptotic proteins Bcl-xL and Mcl-1 is reported. This analogue of a natural sesquiterpenoid dimer meiogynin A was elaborated by a convergent asymmetric synthesis with 36% yield in ten steps.

C. Apel, C. Geny, V. Dumontet, N. Birlirakis, F. Roussi, F. Guéritte, V. C. Pham, H. D. thi Mai, V. H. Nguyen, V. M. Chau, M. Litaudon. J. Nat. Prod. 2014, 77, 1430-1437.
Endiandric acid analogues from Beilschmiedia ferruginea, as dual inhibitors of Bcl-xL/Bak and Mcl-1/Bid Interactions. A rapid screening by 1H and 1H–13C HSQC NMR spectroscopy of EtOAc extracts of Endiandraand Beilschmiedia species allowed the selection of Beilschmiedia ferruginea leaves and flowers extract for a chemical investigation, leading to the isolation of 11 new tetracyclic endiandric acid analogues, named ferrugineic acids A–K (1–11). Their structures were determined by 1D and 2D NMR spectroscopic analysis in combination with HRMS data. These compounds were assayed for Bcl-xL and Mcl-1 binding affinities. Ferrugineic acids B, C, and J (2, 3, and 10) exhibited significant binding affinity for both antiapoptotic proteins Bcl-xL (Ki = 19.2, 12.6, and 19.4 μM, respectively) and Mcl-1 (Ki = 14.0, 13.0, and 5.2 μM, respectively), and ferrugineic acid D (4) showed only significant inhibiting activity for Mcl-1 (Ki = 5.9 μM).

S. Desrat, A. Pujals, C. Colas, J. Dardenne, C. Gény, L. Favre, V. Dumontet, B. I. Iorga, M. Litaudon, M. Raphaël, J. Wiels, F. Roussi. Bioorg. Med. Chem. Lett. 2014, 24, 5086-5088.
Pro-apoptotic meiogynin A derivatives that target Bcl-xL and Mcl-1. The biological evaluation of a natural sesquiterpene dimer meiogynin A 1, is described as well as that of five non-natural analogues. Although active on a micromolar range on the inhibition of Bcl-xL/Bak and Mcl-1/Bid interaction, meiogynin A 1 is not cytotoxic on three cell lines that overexpress Bcl-xL and Mcl-1. Contrarily, one of its analogues 6 with an inverted configuration on the side chain and an aromatic moiety replacing the cyclohexane ring was active on both target proteins, cytotoxic on a micromolar range and was found to induce apoptosis through a classical pathway.
DOI : 10.1016/j.bmcl.2014.09.004

M. N. Azmi, C. Gény, A. Leverrier, M. Litaudon, V. Dumontet, N. Birlirakis, F. Guéritte, K. H. Leong, S. N. Abd. Halim, K. Mohamad and K. Awang Molecules 2014, 19, 1732-1747.
Kingianic Acids A–G, Endiandric Acid Analogues from Endiandra kingiana. A phytochemical investigation of the methanolic extract of the bark of Endiandra kingiana led to the isolation of seven new tetracyclic endiandric acid analogues, kingianic acids A–G (1–7), together with endiandric acid M (8), tsangibeilin B (9) and endiandric acid (10). Their structures were determined by 1D- and 2D-NMR analysis in combination with HRMS experiments. The structure of compounds 9 and 10 were confirmed by single-crystal X-ray diffraction analysis. These compounds were screened for Bcl-xL and Mcl-1 binding affinities and cytotoxic activity on various cancer cell lines. Compound 5 showed moderate cytotoxic activity against human colorectal adeno-carcinoma (HT-29) and lung adenocarcinoma epithelial (A549) cell lines, with IC50 values in the range 15–17 µM, and compounds 3, 6 and 9 exhibited weak binding affinity for the anti-apoptotic protein Mcl-1.
DOI : 10.3390/molecules19021732

J. Dardenne, S. Desrat, F. Guéritte, F. Roussi. Eur. J. Org. Chem. 2013, 11, 2116-2122.
Asymmetric Synthesis of Two Analogues of Meiogynin A. The efficient and asymmetric synthesis of two analogues of meiogynin A, a natural sesquiterpenoid dimer that was recently isolated by our group, is reported. The key reaction was a highly selective intermolecular Diels–Alder reaction between an aromatic triene and two chiral dienes. This convergent synthesis maximizes the atom economy concept, as the expected compounds were obtained in only eight steps without any protecting groups.

Composés à visée antitumorale
O. Gherbovet, M. C. Garcia Alvarez, J. Bignon, S. Thoret, F. Roussi. J. Med. Chem. 2016, 59, 10774-10780.
Original Vinca Derivatives : From P‐Glycoprotein Substrates to P‐Glycoprotein Inhibitors. The first example of vinca derivatives able to modulate P-glycoprotein (Pgp) efflux activity is reported. They were elaborated in two steps from vinorelbine (VLN) by a modification of the velbenamine moiety. These compounds were able to decrease efficiently Pgp mediated influx and efflux of rhodamine-123 (Rho) and to restore the cytotoxicity of vinorelbine (VLN) and doxorubicin (Dox) on K562R (dox-resistant) cell lines.
DOI : 10.1021/acs.jmedchem.6b00525

O. Gherbovet, P. A. Sanchez-Murcia, M. C. Garcia Alvarez, J. Bignon, S. Thoret, F. Gago, F. Roussi. Org. Biomol. Chem.2015, 13, 5520-5531.
Synthesis and evaluation of hybrid molecules targeting the vinca domain of tubulin. Hybrids of vinca alkaloids and phomopsin A, linked by a glycine pattern, have been synthesized in one or two steps, by an insertion reaction. These compounds have been elaborated in order to interact with both the “vinca site” and the “peptide site” of the vinca domain in tubulin. Two out of three hybrids are potent inhibitors of microtubules assembly and they present good cytotoxicity against different cell lines. Molecular modelling studies show that they could bind, within the vinca domain, in similar spatial regions as that of vinca and phomopsin thanks to the flexibility provided by the glycine linker used to elaborate these hybrids.

O. Gerbovet, F. La Spisa, S. Thoret, M. C. Garcia Alvarez, H. Levaique, J. Bignon, F. Roussi. Bioorg. Med. Chem. Lett. 2015, 25, 1771-1773.
Synthesis and biological evaluation of C-13’ substituted 7’-homo-anhydrovinblastine derivatives. Recent publications highlighted that vinca derivatives either functionalized on C-12′ or enlarged on cycle C′ could be more cytotoxic than vinblastine or vinorelbine, both used in anti-cancer therapy. By combining these two results, nine new 7′-homo-anhydrovinblastine derivatives functionalized on C-13′ were elaborated. The synthesis of key intermediates, their one-step transformation into final products in mild conditions and their biological activities are presented.
DOI : 10.1016/j.bmcl.2015.02.045

O. Gherbovet, C. Coderch, M. C. Garcia Alvarez, J. Bignon, S. Thoret, F. Gueritte, F. Gago, F. Roussi. J. Med. Chem.2014, 57, 5470-5476.
One-Pot Synthesis of Vinca Alkaloids-Phomopsin Hybrids. Hybrids of vinca alkaloids and phomopsin A have been elaborated with the aim of interfering with both the “vinca site” and the “peptide site” of the so-called vinca domain in tubulin. They were synthesized by an efficient one-pot procedure that links directly the octahydrophomopsin lateral chain to the velbenamine moiety of 7’-homo-anhydrovinblastine. In their modeled complexes with tubulin, these hybrids were found to superimpose nicely on the tubulin-bound structures of vinblastine and phomopsin A. This good matching can account for the fact that two of them are very potent inhibitors of microtubules assembly and are cytotoxic against four cancer cell lines.

O. Gherbovet, C. Coderch, M. C. Garcia Alvarez, J. Bignon, S. Thoret, M. T. Martin, F. Gueritte, F. Gago, F. Roussi. J. Med. Chem. 2013, 56, 6088-6100.
Synthesis and Biological Evaluation of a New Series of Highly Functionalized 7’-Homo-Anhydrovinblastine Derivatives. Sixteen new 7’ homo-anhydrovinblastine derivatives were prepared in one or two steps from vinorelbine by means of an original and regiospecific rearrangement and subsequent diastereoselective reduction. This strategy has allowed fast access to a family of vinca alkaloid derivatives with an enlarged and functionalized ring C’. Their synthesis and biological evaluation are reported. One compound (compound 35) is 1.7 times more active than vinorelbine as a tubulin assembly inhibitor. Moreover, some of these compounds are highly cytotoxic and two of them are more potent than vinorelbine on HCT116 and K562 cell lines. Molecular modeling studies, carried out with two of the new vinca derivatives, provide useful hints about how a given functionalization introduced at positions 7’ and 8’ of the C’ ring results in improved binding interactions between one of the new derivatives and the interdimer interface when compared to the parent compound vinblastine.

Composés à visée anti-infectieuse
F. Olivon, S. Remy, G. Grelier, C. Apel, C. Eydoux, J-C. Guillemot, J. Neyts, L. Delang, D. Touboul, F. Roussi, M. Litaudon. J. Nat. Prod. 2019, 82, 2, 330-340.
Antiviral Compounds from Codiaeum peltatum Targeted by a Multi-informative Molecular Networks Approach. From a set of 292 Euphorbiaceae extracts, the use of a molecular networking (MN)-based prioritization approach highlighted three clusters (MN1–3) depicting ions from the bark extract of Codiaeum peltatum. Based on their putative antiviral potential and structural novelty, the MS-guided purification of compounds present in MN1 and MN2 afforded two new daphnane-type diterpenoid orthoesters (DDO), codiapeltines A (1) and B (2), the new actephilols B (3) and C (4), and four known 1,4-dioxane-fused phenanthrene dimers (5–8). The structures of the new compounds were elucidated by NMR spectroscopic data analysis, and the absolute configurations of compounds 1 and 2 were deduced by comparison of experimental and calculated ECD spectra. Codiapeltine B (2) is the first daphnane bearing a 9,11,13-orthoester moiety, establishing a new major structural class of DDO. Compounds 1–8 and four recently reported monoterpenyl quinolones (9–12) detected in MN3 were investigated for their selective activities against chikungunya virus replication and their antipolymerase activities against the NS5 proteins of dengue and zika viruses. Compounds 3–8 exhibited strong inhibitory activities on both dengue and zika NS5 in primary assays, but extensive biological analyses indicated that only actephilol B (3) displayed a specific interaction with the NS5 targets.
DOI: 10.1021/acs.jnatprod.8b00800

M. Esposito, L. F. Nothias-Scaglia, P. Retailleau, J. Costa, F. Roussi, J. Neyts, P. Leyssen, D. Touboul, M. Litaudon, J. Paolini. J. Nat. Prod. 2017, 80, 2051-2059.
Isolation of Premyrsinane, Myrsinane, and Tigliane Diterpenoids from Euphorbia pithyusa Using a Chikungunya Virus Cell-Based Assay and Analogue Annotation by Molecular Networking. Six new premyrsinol esters (1–6) and one new myrsinol ester (8) were isolated from an aerial parts extract of Euphorbia pithyusa, together with a known premyrsinol (7) and two known dideoxyphorbol esters (9 and 10), following a bioactivity-guided purification procedure using a chikungunya virus (CHIKV) cell-based assay. The structures of the new diterpene esters (1–6 and 8) were elucidated by MS and NMR spectroscopic data interpretation. Compounds 1–10 were evaluated against CHIKV replication, and results showed that the 4β-dideoxyphorbol ester 10 was the most active compound, with an EC50 value of 4.0 ± 0.3 μM and a selectivity index of 10.6. To gain more insight into the structural diversity of diterpenoids produced by E. pithyusa, the initial extract and chromatographic fractions were analyzed by LC-MS/MS. The generated data were annotated using a molecular networking procedure and revealed that dozens of unknown premyrsinane, myrsinane, and tigliane analogues were present.
DOI : 10.1021/acs.jnatprod.7b00233

M. Esposito, L-F. Nothias-Scaglia, H. Nedev, J.F. Gallard, P. Leyssen, P. Retailleau, J. Costa, F. Roussi, B.I. Iorga, J. Paolini, M. Litaudon. J. Nat. Prod. 2016, 79, 2873-2882.
Euphorbia dendroides Latex as a Source of Jatrophane Esters : Isolation, Structural Analysis, Conformational Study, and Anti-CHIKV Activity. An efficient process was used to isolate six new jatrophane esters, euphodendroidins J (3), K (5), L (6), M, (8), N (10), and O (11), along with seven known diterpenoid esters, namely, euphodendroidins A (4), B (9), E (1), and F (2), jatrophane ester (7), and 3α-hydroxyterracinolides G and B (12 and 13), and terracinolides J and C (14 and 15) from the latex of Euphorbia dendroides. Their 2D structures and relative config- urations were established by extensive NMR spectroscopic analysis. The absolute configurations of compounds 1, 11, and 15 were determined by X-ray diffraction analysis. Euphodendroidin F (2) was obtained in 18% yield from the diterpenoid ester-enriched extract after two consecutive flash chromatography steps, making it an interesting starting material for chemical synthesis. Euphodendroidins K and L (5 and 6) showed an unprecedented NMR spectroscopic behavior, which was investigated by variable-temperature NMR experiments and molecular modeling. The structure−conformation relationships study of compounds 1, 5, and 6, using DFT-NMR calculations, indicated the prominent role of the acylation pattern in governing the conformational behavior of these jatrophane esters. The antiviral activity of compounds 1−15 was evaluated against Chikungunya virus (CHIKV) replication.
DOI : 10.1021/acs.jnatprod.6b00644

L.A. Peyrat, V. Eparvier, C. Eydoux, J.C. Guillemot, D. Stien, M. Litaudon.Fitoterapia 2016, 112, 9-15.
Chemical diversity and antiviral potential in the pantropical Diospyros genus. A screening using a dengue replicon virus-cell-based assay was performed on 3563 ethyl acetate (EtOAc) extracts from different parts of 1500 plants. The screening led to the selection of species from the genus Diospyros (Ebenaceae), among which 25 species distributed in tropical areas showed significant inhibitory activity on dengue virus replication. A metabolic analysis was conducted from the UPLC-HRMS profiles of 33 biologically active and inactive plant extracts, and their metabolic proximity is presented in the form of a dendrogram. The results of the study showed that chemical similarity is not related to plant species or organ. Overall, metabolomic profiling allowed us to define large groups of extracts, comprising both active and inactive ones. Closely related profiles from active extracts might indicate that the common major components of these extracts were responsible for the antiviral activity, while the comparison of chemically similar active and inactive extracts, will permit to find compounds of interest. Eventually, the phytochemical investigation of D. glans bark EtOAc extract afforded usnic acid and 7 known ursane- and lupane-type triterpenoids, among which 5 were found significantly active against dengue virus replication. The inhibitory potency of these compounds was also evaluated on a DENV-NS5 RNA-dependant RNA polymerase assay.
DOI : 10.1016/j.fitote.2016.04.017

L-A. Peyrat, V. Eparvier, V. Eydoux, J-C. Guillemot, M. Litaudon, D. Stien. Chem. Biodiversity 2016, 14, e1600171.
Betulinic Acid, The First Lupane-Type Triterpenoid Isolated from Both a Phomopsis sp. and Its Host Plant Diospyros carbonaria BENOIST. Following a biological screening using a dengue replicon virus-cell-based assay, Diospyros carbonaria AcOEt extract was investigated, affording six known lupane-type triterpenoids endowed with anti-DENV-2 NS5 polymerase activity. The study of the associated microbial community of this species permitted us to identify 38 endophytes belonging to five different orders. Nine out of these 38 strains showed significant activity on the dengue replicon assay. The chemical investigation of the most active one, Phomopsis sp. SNB-LAP1-7-32, led to the isolation of betulinic acid, an anti-viral secondary metabolite isolated previously from the host plant. This result is the first example of a lupane-type triterpenoid isolated from both an endophyte and its host plant. Its presence in the Phomopsis strain may result from gene transfer and/or specific niche selection.
L-F. Nothias-Scaglia, V. Dumontet, J. Neyts, F. Roussi, J. Costa, P. Leyssen, M. Litaudon, J. Paolini. Fitoterapia 2015, 105, 202-209.
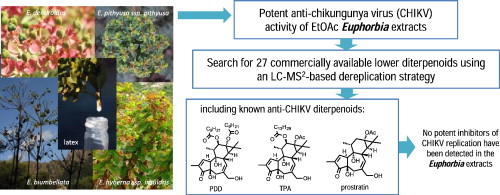
LC-MS2-Based Dereplication of Euphorbia Extracts with Anti-Chikungunya Virus activity. Recently, phorbol esters from Euphorbiaceae have been shown to elicit potent and selective antiviral activity on the replication of Chikungunya virus (CHIKV) in cell culture. With the objective to found new compounds with anti-CHIKV activities, 45 extracts from various plant parts of 11 Mediterranean Euphorbia and one Mercurialis species were evaluated for selective inhibition of CHIKV replication. All EtOAc extracts, especially those prepared from latex, exhibited significant and selective antiviral activity in a Chikungunya virus-cell-based assay. An LC-MS2 dereplication method was then developed to investigate whether known diterpenoids with anti-CHIKV activity, such as the potent anti-CHIKV 12-O-Tetradecanoylphorbol-13-acetate (TPA), phorbol-12,13-didecanoate, and prostratin as well as 24 other commercially available diterpenoids of tigliane-, ingenane-, and daphnane-type for which the anti-CHIKV activity have been established in advance (Nothias-Scaglia et al. 2015), were present in the Euphorbia extracts. Only ingenol-3-mebutate, 13-O-isobutyryl-12-deoxyphorbol-20-acetate, and ingenol-3,20-dibenzoate, all exhibiting weak anti-CHIKV activities, were detected in the EtOAc extracts of E. peplus, E. segetalis ssp. pinea, E. peplus, and E. pithyusa ssp. pithyusa. Given the potent anti-CHIKV activities of these Euphorbia extracts, the present study suggested that their antiviral activities are probably due to untargeted diterpenoids.
DOI : 10.1016/j.fitote.2015.06.021
L-F. Nothias-Scaglia, C. Pannecouque, F. Renucci, L. Delang, J. Neyts, F. Roussi, J. Costa, P. Leyssen, M. Litaudon, J. Paolini. J. Nat. Prod. 2015, 78, 1277-1283.
Antiviral Activity activity of Diterpene esters on Chikungunya virus and HIV replication. Recently, new daphnane, tigliane, and jatrophane diterpenoids have been isolated from various Euphorbiaceae species, of which some have been shown to be potent inhibitors of chikungunya virus (CHIKV) replication. To further explore this type of compound, the antiviral activity of a series of 29 commercially available natural diterpenoids was evaluated. Phorbol-12,13-didecanoate (11) proved to be the most potent inhibitor, with an EC50 value of 6.0 ± 0.9 nM and a selectivity index (SI) of 686, which is in line with the previously reported anti-CHIKV potency for the structurally related 12-O-tetradecanoylphorbol-13-acetate (13). Most of the other compounds exhibited low to moderate activity, including an ingenane-type diterpene ester, compound 28, with an EC50 value of 1.2 ± 0.1 μM and SI = 6.4. Diterpene compounds are known also to inhibit HIV replication, so the antiviral activities of compounds 1–29 were evaluated also against HIV-1 and HIV-2. Tigliane- (4β-hydroxyphorbol analogues 10, 11, 13,15, 16, and 18) and ingenane-type (27 and 28) diterpene esters were shown to inhibit HIV replication in vitro at the nanomolar level. A Pearson analysis performed with the anti-CHIKV and anti-HIV data sets demonstrated a linear relationship, which supported the hypothesis made that PKC may be an important target in CHIKV replication.
DOI : 10.1021/acs.jnatprod.5b00073

F. Olivon, H. Palenzuela, E. Girard-Valenciennes, J. Neyts, C. Pannecouque, F. Roussi, I. Grondin, P. Leyssen, M. Litaudon. J. Nat. Prod. 2015, 78, 1119-1128
Antiviral Activity of Flexibilane and Tigliane:Diterpenoids from Stillingia lineata. J. In an effort to identify new potent and selective inhibitors of chikungunya, HIV-1 and HIV-2 virus replication, the endemic Mascarene species Stillingia lineata was investigated. LC/MS- and bioassay-guided purification of the EtOAc leaves extract using a chikungunya virus-cell-based assay led to the isolation of six new (4-9) and three known (1-3) tonantzitlolones possessing the rare C20-flexibilane skeleton, along with tonantzitloic acid (10), a new linear diterpenoid, and three new (11, 13, and 15) and two known (12 and 14) tigliane-type diterpenoids. The planar structures of the new compounds and their relative configurations were determined by spectroscopic analysis and their absolute configurations were determined through comparison with literature data and from biogenetic considerations. These compounds were investigated for selective antiviral activity against chikungunya virus (CHIKV), Semliki Forest virus, Sindbis virus, and for compounds 11-15, the HIV-1 and HIV-2 viruses. Compounds 12-15 were found to be the most potent and are selective inhibitors of CHIKV, HIV-1, and HIV-2 replication. In particular, compound 14 inhibited CHIKV replication with an EC50 value of 1.2 µM on CHIKV and a selectivity index > 240, while compound 15 inhibited HIV-1 and HIV-2 with EC50 values of 0.043 and 0.018 µM, respectively. It was demonstrated further that potency and selectivity are sensitive to the substitution pattern on the tigliane skeleton. The cytotoxic activities of compounds 1-10 were evaluated against the HCT-116, MCF-7, and PC3 cancer cell lines.
DOI : 10.1021/acs.jnatprod.5b00116

L-F. Nothias-Scaglia, P. Retailleau, J. Paolini, C. Pannecouque, J. Neyts, V. Dumontet, F. Roussi, P. Leyssen, J. Costa, M. Litaudon. J. Nat. Prod.2014, 77, 1505-1512.
Jatrophanes diterpenes as inhibitors of chikungunya virus replication : Structure-activity relationship and discovery of a potent lead. Bioassay-guided purification of an EtOAc extract of the whole plant of Euphorbia amygdaloidesssp. semiperfoliata using a chikungunya virus-cell-based assay led to the isolation of six new (1–4, 9, and 10) and six known (5–7, 8, 11, and 12) jatrophane esters. Their planar structures and relative configurations were determined by extensive spectroscopic analysis, and their absolute configurations by X-ray analysis. These compounds were investigated for selective antiviral activity against chikungunya virus (CHIKV), Semliki Forest virus, Sindbis virus, and HIV-1 and HIV-2 viruses. Compound 3 was found to be the most potent and selective inhibitor of the replication of CHIKV and of HIV-1 and HIV-2 (EC50 = 0.76, IC50 = 0.34 and 0.043 μM, respectively). A preliminary structure–activity relationship study demonstrated that potency and selectivity are very sensitive to the substitution pattern on the jatrophane skeleton. Although replication strategies of CHIK and HIV viruses are quite different, the mechanism of action by which these compounds act may involve a similar target for both viruses. The present results provide additional support for a previous hypothesis that the anti-CHIKV activity could involve a PKC-dependent mechanism.

N. Corlay, L. Delang, E. Girard-Valenciennes, J. Neyts, P. Clerc, J. Smadja, F. Guéritte, P. Leyssen, M. Litaudon. Fitoterapia 2014, 97, 87-91.
Tigliane diterpenes from Croton mauritianus as inhibitors of chikungunya virus replication. A bioassay-guided purification of an EtOAc extract of the leaves of Croton mauritianususing a chikungunya virus-cell-based assay led to the isolation of 12-O-decanoylphorbol-13-acetate (1) and the new 12-O-decanoyl-7-hydroperoxy-phorbol-5-ene-13-acetate (2), along with loliolide, vomifoliol, dehydrovomifoliol, annuionone D and bluemol C. The planar structure and the relative configuration of compound 2 were elucidated based on spectroscopic analysis, including 1D- and 2D-NMR experiments, mass spectrometry, and comparison with literature data. Compounds 1 and 2 inhibited chikungunya virus-induced cell death in cell culture with EC50s of 2.4 ± 0.3 and 4.0 ± 0.8 μM, respectively.
DOI : 10.1016/j.fitote.2014.05.015

M. Bourjot, P. Leyssen, J. Neyts, V. Dumontet, M. Litaudon. Molecules. 2014, 19, 3617-3627.
Trigocherrierin A, a potent Inhibitor of chikungunya virus replication. Trigocherrierin A (1) and trigocherriolide E (2), two new daphnane diterpenoid orthoesters (DDOs), and six chlorinated analogues, trigocherrins A, B, F and trigocherriolides A–C, were isolated from the leaves of Trigonostemon cherrieri. Their structures were identified by mass spectrometry, extensive one- and two-dimensional NMR spectroscopy and through comparison with data reported in the literature. These compounds are potent and selective inhibitors of chikungunya virus (CHIKV) replication. Among the DDOs isolated, compound 1 exhibited the strongest anti-CHIKV activity (EC50 = 0.6 ± 0.1 µM, SI = 71.7).
DOI : 10.3390/molecules19033617

M. Bourjot, L. Delang, V. H. Nguyen, J. Neyts, F. Guéritte, P. Leyssen, M. Litaudon. J. Nat. Prod. 2012, 75, 2183-2187.
Prostratin and 12-O-Tetradecanoylphorbol 13-acetate Are Potent and Selective Inhibitors of Chikungunya Virus Replication. A chemical study of the Vietnamese plant species Trigonostemon howii led to the isolation of a new tigliane-type diterpenoid, trigowiin A (1), along with several known coumarins and phenylpropanoids. The planar structure and the relative configuration of compound 1 were elucidated based on spectroscopic analysis, including 1D- and 2D-NMR experiments, mass spectrometry, and comparison with literature data. Trigowiin A (1) exhibited moderate antiviral activity in a virus-cell-based assay for Chikungunya virus (CHIKV). Since the structure of compound 1 is closely related to those of well-known tigliane diterpenoids such as prostratin(2), phorbol (3), 12-O-tetradecanoylphorbol 13-acetate (TPA) (4), and 4α-TPA (5), the antiviral activity of the latter compounds was also evaluated against CHIKV, as well as in virus-cell-based assays of two additional members of the genus Alphavirus (Sindbis virus, SINV, and Semliki forest virus, SFV). Whereas prostratin inhibited CHIKV replication with a moderate EC50 of 2.6 μM and a selectivity index (SI) approximating 30, compound 4 proved to be an extremely potent inhibitor, with an EC50 of ∼3 nM and a SI near 2000. Interestingly, no or very little activity was observed on the replication of SINV and SFV.

P-M. Allard, P. Leyssen, M-T. Martin, M. Bourjot, V. Dumontet, C. Eydoux, J-Cl. Guillemot, B. Canard, C. Poullain, F. Guéritte, M. Litaudon. Phytochemistry 2012, 84, 160-168.
Antiviral chlorinated daphnane diterpenoid orthoesters from the bark and wood of Trigonostemon cherrieri. The chemical study of the bark and the wood of Trigonostemon cherrieri, a rare endemic plant of New Caledonia, led to the isolation of a series of highly oxygenated daphnane diterpenoid orthoesters (DDO) bearing an uncommon chlorinated moiety : trigocherrins A–F and trigocherriolides A–D. Herein, we describe the isolation and structure elucidation of the DDO (trigocherrins B–F and trigocherriolides A–D). We also report the antiviral activity of trigocherrins A, B and F (1, 2 and 6) and trigocherriolides A, B and C (7–9) against various emerging pathogens : chikungunya virus (CHIKV), Sindbis virus (SINV), Semliki forest virus (SFV) and dengue virus (DENV).
DOI : 10.1016/j.phytochem.2012.07.023

M. Bourjot, P. Leyssen, C. Eydoux, J-C. Guillemot, B. Canard, P. Rasoanaivo, F. Guéritte, M. Litaudon. Fitoterapia 2012, 83, 1076-1080.
Chemical constituents of Anacolosa pervilleana and their antiviral activities. In an effort to identify novel inhibitors of Chikungunya (CHIKV) and Dengue (DENV) virus replication, a systematic study with 820 ethyl acetate extracts of Madagascan plants was performed in a virus-cell-based assay for CHIKV and a DENV NS5 RNA-dependant RNA polymerase (RdRp) assay. The extract obtained from the leaves of Anacolosa pervilleana was selected for its significant activity in both assays. One new (E)-tridec-2-en-4-ynedioic acid named anacolosine (1), together with three known acetylenic acids, the octadeca-9,11,13-triynoic acid (2), (13E)-octadec-13-en-9,11-diynoic acid (3), (13E)-octadec-13-en-11-ynoic acid (4), two terpenoids, lupenone (5) and β-amyrone (6), and one cyanogenic glycoside, (S)-sambunigrin (7) were isolated. Their structures were elucidated by comprehensive analyses of NMR spectroscopy and mass spectrometry data. The inhibitory potency of these compounds was evaluated on CHIKV, DENV RdRp and West-Nile polymerase virus (WNV RdRp). Both terpenoids showed a moderate activity against CHIKV (EC50 77 and 86 μM, respectively) and the acetylenic acids produced IC50 values around 3 μM in the DENV RdRp assay.
DOI : 10.1016/j.fitote.2012.05.004

M. Bourjot, P. Leyssen, C. Eydoux, J-C. Guillemot, B. Canard, P. Rasoanaivo, F. Guéritte, and M. Litaudon. J. Nat. Prod. 2012, 75, 752-758.
Flacourtosides A-F, phenolic glycosides isolated from Flacourtia ramontchi. In an effort to identify novel inhibitors of chikungunya (CHIKV) and dengue (DENV) virus replication, a systematic study with 820 ethyl acetate extracts of madagascan plants was performed in a virus-cell-based assay for CHIKV, and a DENV NS5 RNA-dependent RNA polymerase (RdRp) assay. The extract obtained from the stem bark of Flacourtia ramontchi was selected for its significant activity in both assays. Six new phenolic glycosides, named flacourtosides A–F (1–6), phenolic glycosides itoside H, xylosmin, scolochinenoside D, and poliothrysoside, and betulinic acid 3β-caffeate were obtained using the bioassay-guided isolation process. Their structures were elucidated by comprehensive analyses of NMR spectroscopic and mass spectrometric data. Even though several extracts and fractions showed significant selective antiviral activity in the CHIKV virus-cell-based assay, none of the purified compounds did. However, in the DENV RNA polymerase assay, significant inhibition was observed with betulinic acid 3β-caffeate (IC50 = 0.85 ± 0.1 μM) and to a lesser extent for the flacourtosides A and E (1 and 5, respectively), and scolochinenoside D (IC50 values ∼10 μM).

P-M. Allard, M-T. Martin, E. Tran Huu Dau, P. Leyssen, F. Guéritte, and M. Litaudon. Org. Lett. 2012, 14, 342-345.
Trigocherrin A, the first natural chlorinated diterpene orthoester from Trigonostemon cherrieri. Trigocherrin A, a chlorinated and highly oxygenated daphnane diterpenoid orthoester (DDO), was isolated from the bark of Trigonostemon cherrieri. Trigocherrin A is the first example of a naturally occurring halogenated DDO. Its structure was elucidated by comprehensive analysis of NMR spectroscopic data, and its absolute configuration was determined by comparison of experimental and theoretically calculated ECD spectra. Trigocherrin A exhibited a potent and selective effect against Chikungunya virus in Vero cells.

Composés à visée anti-infectieuse
M. A. Beniddir, M-T. Martin, M-E. Tran Huu Dau, P. Grellier, P. Rasoanaivo, F. Guéritte, M. Litaudon. Org. Lett. 2012, 14, 4162-4165.
Goniomedines A and B : Unprecedented Bisindole Alkaloids Formed through Fusion of Two Indole Moieties via a Dihydropyran Unit. Two novel bisindole alkaloids, goniomedines A (1) and B (2), possessing an unprecedented quebrachamine–pleioarpamine-type skeleton, in which indole moieties are fused via a dihydropyran unit, were isolated from the stem bark of Gonioma malagasy. The structures were elucidated by comprehensive analysis of MS and NMR spectroscopic data. Their absolute configurations were deduced following the comparison of experimental and theoretically calculated ECD spectra and through biogenetic considerations. Goniomedine B (2) exhibited moderate activity against Plasmodium falciparum.

M. A. Beniddir, P. Grellier, P. Rasoanaivo, P. M. Loiseau, C. Bories, V. Dumontet, F. Guéritte, M. Litaudon. Eur. J. Org. Chem. 2012, 5, 1039-1046.
Diarylheptanoid glucosides from Pyrostria major and their antiprotozoal activities. Eight new diarylheptanoid glucosides 1–8 have been isolated from the leaves of Pyrostria major (A. Rich & DC.) Cavaco [syn. Canthium major (A. Rich & DC.)] Cavaco. Their structures were determined by 1D and 2D NMR spectroscopic and HRMS analyses. The absolute configurations of the diarylheptanoids 1–4 were determined to be 3S and 5S by the application of the CD exciton chirality method to the corresponding 3,5-bis(p-bromobenzoyl) (1–3) and 3,5-bis(p-dimethylaminobenzoyl) (4) derivatives. Their antiparasitic activities against Plasmodium falciparum, Leishmania donovani (amastigote forms), and Trypanosoma brucei (trypomastigote bloodstream forms) as well as their cytotoxic activities against the HL-60, KB, and MRC5 cell lines of naturally occurring diarylheptanoid glucosides and their derivatives are reported.

Composés à visée anti-infectieuse
N. Corlay, M. Lecsö-Bornet, E. Leborgne, F. Blanchard, X. Cachet, J. Bignon, F. Roussi, M-J. Butel, K. Awang, and M. Litaudon. J. Nat. Prod. 2015, 78, 1348-1356.
Antibacterial labdane diterpenoids from Vitex vestita. A large-scale in vitro screening of tropical plants using an antibacterial assay permitted the selection of several species with significant antibacterial activities. Bioassay-guided purification of the dichloromethane extract of the leaves of the Malaysian species Vitex vestita, led to the isolation of six new labdane-type diterpenoids, namely, 12-epivitexolide A (2), vitexolides B and C (3 and 4), vitexolide E (8), and vitexolins A and B (5 and 6), along with six known compounds, vitexolides A (1) and D (7), acuminolide (9), 3β-hydroxyanticopalic acid (10), 8α-hydroxyanticopalic acid (11), and 6α-hydroxyanticopalic acid (12). Their structures were elucidated on the basis of 1D and 2D NMR analyses and HRMS experiments. Both variable-temperature NMR spectroscopic studies and chemical modifications were performed to investigate the dynamic epimerization of the γ-hydroxybutenolide moiety of compounds 1–4. Compounds were assayed against a panel of 46 Gram-positive strains. Vitexolide A (1) exhibited the most potent antibacterial activity with minimal inhibitory concentration values ranging from 6 to 96 μM, whereas compounds 2 and 6–9 showed moderate antibacterial activity. The presence of a β-hydroxyalkyl-γ-hydroxybutenolide subunit contributed significantly to antibacterial activity. Compounds 1–4 and 6–9 showed cytotoxic activities against the HCT-116 cancer cell line (1 < IC50s < 10 μM) and human fetal lung fibroblast MRC5 cell line (1 < IC50s < 10 μM for compounds 1, 2, 7, 8, and 9).
DOI : 10.1021/acs.jnatprod.5b00206

F. Olivon, L-F. Nothias, V. Dumontet, P. Retailleau, S. Berger, G. Ferry, W. Cohen, B. Pfeiffer, J. A. Boutin, E. Scalbert, F. Roussi, M. Litaudon. J. Nat. Prod. 2018, 81, 1610-1618.
Natural Inhibitors of the RhoA–p115 Complex from the Bark of Meiogyne baillonii. In an effort to find potent natural inhibitors of RhoA and p115 signaling G-proteins, a systematic in vitro evaluation using enzymatic and plasmonic resonance assays was undertaken on 11 317 plant extracts. The screening procedure led to the selection of the New Caledonian endemic species Meiogyne baillonii for a chemical investigation. Using a bioguided isolation procedure, three enediyne-γ-butyrolactones (1-3) and two enediyne-γ-butenolides (4 and 5), named sapranthins H-L, respectively, two enediyne carboxylic acid (6 and 7), two depsidones, stictic acid (8) and baillonic acid (9), aristolactams AIa and AIIa (10 and 11), and two aporphines, dehydroroemerine (12) and noraristolodione (13), were isolated from the ethyl acetate extract of the bark. The structures of the new compounds (1-6, 9, and 11) and their relative configurations were established by NMR spectroscopic analysis and by X-ray diffraction analysis for compound 9. Only stictic acid (8) exhibited a significant inhibiting activity of the RhoA-p115 complex, with an EC50 value of 0.19 ± 0.05 mM. This is the first time that a natural inhibitor of the complex RhoA-p115’s activity was discovered from an HTS performed over a collection of higher plant extracts. Thus, stictic acid (8) could be used as the first reference compound inhibiting the interaction between RhoA and p115.
DOI : 10.1021/acs.jnatprod.8b00209

M. Esposito, S. Nim, L. F. Nothias-Scaglia, J. F. Gallard, M. Kaur Rawal, J. Costa, F. Roussi, R. Prasad, A. Di Pietro, J. Paolini, M. Litaudon. J. Nat. Prod. 2017, 80, 479-487.
Evaluation of Jatrophane Esters from Euphorbia spp. as Modulators of Candida albicans Multidrug Transporters. Twenty-nine jatrophane esters and one lathyrane diterpenoid ester isolated from Euphorbia species were evaluated for their capacity to inhibit drug-efflux activities of the primary ABC transporter CaCdr1p and the secondary MFS transporter CaMdr1p of Candida albicans, in yeast strains overexpressing the corresponding transporter. These diterpenoid esters were obtained from Euphorbia semiperfoliata, E. insularis, and E. dendroides and included five new compounds, euphodendroidins P–T. The jatrophane esters 12 and 23 were found to inhibit the efflux of Nile Red (NR) mediated by the two multidrug transporters, at 85–64% for CaCdr1p and 79–65% for CaMdr1p. In contrast, compound 21 was selective for CaCdr1p and induced a strong inhibition (92%), whereas compound 8 was selective for CaMdr1p, with a 74% inhibition. It was demonstrated further that potency and selectivity are sensitive to the substitution pattern on the jatrophane skeleton. However, these compounds were not transported and showed no synergism with fluconazole cytotoxicity.
DOI : 10.1021/acs.jnatprod.6b00990

M. A. Beniddir, E. Le Borgne, B. I. Iorga, N. Loaëc, O. Lozach, L. Meijer, K. Awang, and M. Litaudon. J. Nat. Prod. 2014, 77, 1117-1122.
Acridone alkaloids from Glycosmis chlorosperma as DYRK1A inhibitors. Two new acridone alkaloids, chlorospermines A and B (1 and 2), were isolated from the stem bark of Glycosmis chlorosperma, together with the known atalaphyllidine (3) and acrifoline (4), by means of bioguided isolation using an in vitro enzyme assay against DYRK1A. Acrifoline (4) and to a lesser extent chlorospermine B (2) and atalaphyllidine (3) showed significantinhibiting activity on DYRK1A with IC50’s of 0.075, 5.7, and 2.2 μM, respectively. Their selectivity profile was evaluated against a panel of various kinases, and molecular docking calculations provided structural details for the interaction between these compounds and DYRK1A.

C. Apel, V. Dumontet, O. Lozach, L. Meijer, F. Guéritte, M. Litaudon. Phytochem. Lett. 2012, 5, 814-818.
Phenanthrene derivatives from Appendicula reflexa as new CDK1/cyclin B inhibtors. The chemical investigation of the epiphytic orchid Appendicula reflexa led to the isolation of one new phenanthrene, 3,4,6,7-tetramethoxyphenanthrene-2,8-diol (1) and one new bisphenanthrene ether, named blestrin E (6), together with the known monomeric phenanthrenes, nudol (2), 3,4,6-trimethoxyphenanthrene-2,7-diol (3), coelonin (4) and 6-methoxycoelonin (5). Their structural elucidation was established on the basis of spectroscopic data analysis. The monomeric phenanthrenes 1 and 3 showed moderate cytotoxic activities against KB, MCF7 and K562 cells, and potent inhibiting activity on CDK1/cyclin B (IC50s = 0.07 and 0.2 μM, respectively).
DOI : 10.1016/j.phytol.2012.09.008

C. Levrier, B. Kiremire, F. Guéritte, M. Litaudon. Fitoterapia 2012, 83, 660-664.
Toxicarioside M, a new cytotoxic 10β-hydroxy-19-nor-cardenolide from Antiaris toxicaria. A new 10β-hydroxy-19-nor-cardenolide, named toxicarioside M (1), was isolated from the trunk bark of Antiaris toxicaria (Pers.) Lesch (Moraceae), along with six known cardenolides (convallatoxin (2), convallatoxol (3), convalloside (4), 3-O-ß-D-xylopyranosylstrophanthidin (5), glucostrophanthidin (6) and strophanthidin (7)). Their structures were elucidated on the basis of HR-MSn analysis, spectroscopic methods (IR, UV, 1D and 2D NMR) and by comparison with data reported in the literature. The cardenolides were evaluated for their cytotoxic activity against KB, HCT-116, SF-268, MCF-7, HL-60, PC-3 and MRC-5 cell lines.
DOI : 10.1016/j.fitote.2012.02.001

Développement méthodologique en chimie analytique
F. Olivon, N. Elie, G. Grelier, F. Roussi, M. Litaudon, D. Touboul. Anal. Chem. 2018, 90, 23, 13900-13908.
MetGem software for the generation of molecular networks based on t-SNE algorithm. Molecular Networking (MN) is becoming a standard bioinformatics tool in the metabolomic community. Its paradigm is based on the observation that compounds with high chemical similarity share comparable MS² fragmentation pathways. To afford a clear separation between MS2 spectra clusters only the most relevant similarity scores are selected using dedicated filtering steps requiring time-consuming parameters optimization. Depending on the filtering values selected, some scores are arbitrarily deleted and a part of the information is ignored. The difficulty of creating a reliable representation of MS² spectra datasets can be solved using algorithms developed for dimensionality reduction and pattern recognition purposes, such as the t-distributed stochastic neighbor embedding (t-SNE). This multivariate embedding method gives a particular attention for local details by using non-linear outputs to represent the entire data space. To overcome the limitations inherent to the GNPS workflow and the networking architecture, we developed MetGem. Our software allows the parallel investigation of two complementary representations of the raw dataset, one based on a classic GNPS-style MN and another one based on the t-SNE algorithm. The t-SNE graph preserves the interactions between related groups of spectra while the MN output allows an unambiguous separation of clusters. Additionally, almost all parameters can be tuned in real time and new networks can be generated within few seconds for small datasets. With the development of this unified interface (https://metgem.github.io), we fulfilled the need of a dedicated, user-friendly, local software for MS² comparison and spectral networks generation.
DOI : 10.1021/acs.analchem.8b03099

F. Olivon, C. Apel, P. Retailleau, P-M. Allard, J.L. Wolfender, D. Touboul, F. Roussi, M. Litaudon, S. Desrat. Org. Chem. Front. 2018, 5, 2171-2178.
Searching for original natural products by molecular networking : detection, isolation and total synthesis of chloroaustralasines. With the aim of isolating structurally original natural products, a molecular networking (MN)-based prioritisation approach has been developed and applied to a collection of 292 plant extracts. It led to the selection of a sample-specific cluster of ions detected in the bark extract of Codiaeum peltatum. The MN-guided purification of the targeted compounds afforded four unprecedented chlorinated monoterpenyl quinolones named chloroaustralasines A–C and isochloroaustralasine A. Faced with inconsistent spectral data of some previously reported quinolones, the total synthesis of the corresponding dihydroxy and chlorohydrin compounds was undertaken. The desired products were obtained in three steps, allowing the structural reassignment of two erioaustralasines. The chloroperoxidase-mediated hydroxychlorination reaction developed for the synthesis of the chlorinated quinolone showed that such complex molecules could be good substrates for this enzyme and, at the same time, raised the question of the biosynthetic origin of the non-artefactual chlorohydrin moiety.

L-F. Nothias, M. Nothias-Esposito, R. da Silva, M. Wang, I. Protsyuk, Z. Zhang, A. Sarvepalli, P. Leyssen, D. Touboul, J. Costa, J. Paolini, T. Alexandrov, M. Litaudon, P. C. Dorrestein. J. Nat. Prod. 2018, 81, 758-767.
Bioactivity-Based Molecular Networking for the Discovery of Drug Leads in Natural Product Bioassay-Guided Fractionation. It is a common problem in natural product therapeutic lead discovery programs that despite good bioassay results in the initial extract, the active compound(s) may not be isolated during subsequent bioassay-guided purification. Herein, we present the concept of bioactive molecular networking to find candidate active molecules directly from fractionated bioactive extracts. By employing tandem mass spectrometry, it is possible to accelerate the dereplication of molecules using molecular networking prior to subsequent isolation of the compounds, and it is also possible to expose potentially bioactive molecules using bioactivity score prediction. Indeed, bioactivity score prediction can be calculated with the relative abundance of a molecule in fractions and the bioactivity level of each fraction. For that reason, we have developed a bioinformatic workflow able to map bioactivity score in molecular networks and applied it for discovery of antiviral compounds from a previously investigated extract of Euphorbia dendroides where the bioactive candidate molecules were not discovered following a classical bioassay-guided fractionation procedure. It can be expected that this approach will be implemented as a systematic strategy, not only in current and future bioactive lead discovery from natural extract collections but also for the reinvestigation of the untapped reservoir of bioactive analogues in previous bioassay-guided fractionation efforts.
DOI : 10.1021/acs.jnatprod.7b00737

L-F. Nothias, S. Boutet-Mercey, X. Cachet, E. De La Torre, L. Laboureur, J-F. Gallard, P. Retailleau, A. Brunelle, P. C. Dorrestein, J. Costa, L. M. Bedoya, F. Roussi, P. Leyssen, J. Alcami, J. Paolini, M. Litaudon, D. Touboul. J. Nat. Prod. 2017, 80, 2620-2629.
Environmentally Friendly Procedure Based on Supercritical Fluid Chromatography and Tandem Mass Spectrometry Molecular Networking for the Discovery of Potent Antiviral Compounds from Euphorbia semiperfoliata. A supercritical fluid chromatography-based targeted purification procedure using tandem mass spectrometry and molecular networking was developed to analyze, annotate, and isolate secondary metabolites from complex plant extract mixture. This approach was applied for the targeted isolation of new antiviral diterpene esters from Euphorbia semiperfoliata whole plant extract. The analysis of bioactive fractions revealed that unknown diterpene esters, including jatrophane esters and phorbol esters, were present in the samples. The purification procedure using semipreparative supercritical fluid chromatography led to the isolation and identification of two new jatrophane esters (13 and 14) and one known (15) and three new 4-deoxyphorbol esters (16–18). The structure and absolute configuration of compound 16 were confirmed by X-ray crystallography. This compound was found to display antiviral activity against Chikungunya virus (EC50 = 0.45 μM), while compound 15 proved to be a potent and selective inhibitor of HIV-1 replication in a recombinant virus assay (EC50 = 13 nM). This study showed that a supercritical fluid chromatography-based protocol and molecular networking can facilitate and accelerate the discovery of bioactive small molecules by targeting molecules of interest, while minimizing the use of toxic solvents.
DOI : 10.1021/acs.jnatprod.7b00113

F. Olivon, P.M. Allard, A. Koval, D. Righi, G. Genta-Jouve, J. Neyts, C. Apel, C. Pannecouque, L.F. Nothias, X. Cachet, L. Marcourt, F. Roussi, V. L. Katanaev, D. Touboul, J.L. Wolfender, M. Litaudon. ACS Chem. Biol.2017, 12, 2644–2651.
Bioactive natural products prioritization using massive multi-informational molecular networks. Natural products represent an inexhaustible source of novel therapeutic agents. Their complex and constrained three-dimensional structures endow these molecules with exceptional biological properties, thereby giving them a major role in drug discovery programs. However, the search for new bioactive metabolites is hampered by the chemical complexity of the biological matrices in which they are found. The purification of single constituents from such matrices requires such a significant amount of work that it should ideally be performed only on molecules of high potential value (i.e. chemical novelty and biological activity). Recent bioinformatics approaches based on state-of the art mass spectrometry metabolite profiling methods are beginning to address the complex task of chemical identification of individual metabolites within complex mixtures. However, in parallel to these developments, methods providing information on the bioactivity potential of natural products prior to their isolation are still lacking and are of key interest to target the isolation of valuable natural products only. In the present investigation, we propose an integrated analysis strategy for bioactive natural products prioritization. Our approach uses massive molecular networks embedding various informational layers (bioactivity and taxonomical data) to highlight potentially bioactive scaffolds within the chemical diversity of crude extracts collections. We exemplify this workflow by targeting the isolation of predicted active and non-active metabolites from two botanical sources (Bocquillonia nervosa and Neoguillauminia cleopatra) against two biological targets (Wnt signaling pathway and chikungunya virus replication). Eventually, the detection and isolation processes of a daphnane diterpene orthoester and four 12-deoxyphorbols inhibiting the Wnt signaling pathway and exhibiting potent antiviral activities against CHIKV virus are detailed. Combined with efficient metabolite annotation tools, this bioactive natural products prioritization pipeline proves to be efficient. Implementation of this approach in drug discovery programs based on natural extract screening should speed up and rationalize the isolation of bioactive natural products.
DOI : 10.1021/acschembio.7b00413

F. Olivon, G. Grelier, F. Roussi, M. Litaudon, D. Touboul. Anal. Chem. 2017, 89, 7836–7840.
MZmine 2 Data-Preprocessing To Enhance Molecular Networking Reliability. Molecular networking is becoming more and more popular into the metabolomic community to organize tandem mass spectrometry (MS2) data. Even though this approach allows the treatment and comparison of large data sets, several drawbacks related to the MS-Cluster tool routinely used on the Global Natural Product Social Molecular Networking platform (GNPS) limit its potential. MS-Cluster cannot distinguish between chromatography well-resolved isomers as retention times are not taken into account. Annotation with predicted chemical formulas is also not implemented and semiquantification is only based on the number of MS2 scans. We propose to introduce a data-preprocessing workflow including the preliminary data treatment by MZmine 2 followed by a homemade Python script freely available to the community that clears the major previously mentioned GNPS drawbacks. The efficiency of this workflow is exemplified with the analysis of six fractions of increasing polarities obtained from a sequential supercritical CO2 extraction of Stillingia lineata leaves.
DOI : 10.1021/acs.analchem.7b01563

T. Péresse, N. Elie, D. Touboul, V.C. Pham, V. Dumontet, F. Roussi, M. Litaudon, A. Brunelle. Anal. Chem. 2017, 89, 9247–9252.
Dual Beam Depth Profiling and Imaging with Argon and Bismuth Clusters of Prenylated Stilbenes on Glandular Trichomes of Macaranga vedeliana. Using a time-of-flight secondary ion mass spectrometer equipped with an argon cluster ion for sputtering and a bismuth liquid metal ion source for analysis, both surfaces of leaves and fruits of Macaranga vedeliana, an endemic New Caledonian species, have been for the first time analyzed by a dual beam depth profiling. To prevent in-vacuum evaporation of the liquid content of the small glandular trichomes covering fruits and leaves surfaces and also to be able to analyze their liquid content while preventing any sublimation of the latter, the samples were kept frozen during the whole experiment using a nitrogen cooled sample holder. Thus, it was possible to demonstrate that vedelianin, an active metabolite of the family of prenylated stilbenes named schweinfurthins, is only located in these glandular trichomes.
DOI : 10.1021/acs.analchem.7b02020

F. Olivon, F. Roussi, M. Litaudon, D. Touboul. Anal. Bioanal. Chem. 2017, 409, 5767–5778.
Optimized experimental workflow for tandem mass spectrometry molecular networking in metabolomics. New omics sciences generate massive amounts of data, requiring to be sorted, curated, and statistically analyzed by dedicated software. Data-dependent acquisition mode including inclusion and exclusion rules for tandem mass spectrometry is routinely used to perform such analyses. While acquisition parameters are well described for proteomics, no general rule is currently available to generate reliable metabolomic data for molecular networking analysis on the Global Natural Product Social Molecular Networking platform (GNPS). Following on from an exploration of key parameters influencing the quality of molecular networks, universal optimal acquisition conditions for metabolomic studies are suggested in the present paper. The benefit of data pre-clustering before initiating large datasets for GNPS analyses is also demonstrated. Moreover, an efficient workflow dedicated to Agilent Technologies instruments is described, making the dereplication process easier by unambiguously distinguishing isobaric isomers eluted at different retention times, annotating the molecular networks with chemical formulas, and giving access to semi-quantitative data. This specific workflow foreshadows future developments of the GNPS platform.
DOI : 10.1007/s00216-017-0523-3
C. Remeur, E. Le Borgne, L. Gauthier, R. Grougnet, B. Deguin, C. Poullain, M. Litaudon. Phytochem. Anal. 2016, 28, 242-246.
HPLC-ELSD Quantification and Centrifugal Partition Chromatography Isolation of 8-O- Acetylharpagide from Oxera coronata (Lamiaceae). Introduction – Iridoid glycosides possess highly functionalised monoterpenoid aglycon with several contiguous stereocentres. For the most common, they are often present in quantities reaching several percentage of the fresh plant weight, and thus they may be regarded as starting material for the synthesis of a number of new chiral and bioactive molecules. Objective – To quantify and to isolate 8-O-acetylharpagide (AH) from several extracts of Oxera coronata R.P.J. de Kok, a Lamiaceae species endemic to New Caledonia, using HPLC-ELSD (evaporative light scattering detector) and centrifugal partition chromatography (CPC). Methodology – Oxera coronata produces high amounts of AH in leaves, twigs and fruits. Water and methanol extracts of these plant parts were prepared. The content of AH in each extract was quantified by HPLC-ELSD, using acetonitrile–water (+0.1% formic acid) gradient elution. The HPLC method was validated for precision, linearity, limit of detection (LOD), limit of quanti- fication (LOQ) and accuracy. A ternary solvent system ethyl acetate/n-propanol/water (3:2:5, v/v/v) was selected and applied to recover the target compound using Spot CPC from the leaves aqueous extract. Results – HPLC-ELSD analysis followed by CPC purification led to the efficient isolation of AH from O. coronata leaves aqueous extract. Conclusion – HPLC-ELSD has proven to be a well-adapted detection and quantification method for iridoid glycosides, while CPC confirmed to be an efficient technique for the isolation of polar compounds. Copyright © 2016 John Wiley & Sons, Ltd.
P.M. Allard, T. Péresse, J. Bisson, K. Gindro, L. Marcourt, V. Cuong Pham, F. Roussi, M. Litaudon, J.L. Wolfender. Anal. Chem. 2016, 88, 3317-3323.
Integration of Molecular Networking and In-Silico MS/MS Fragmentation for Natural Products Dereplication. Dereplication represents a key step for rapidly identifying known secondary metabolites in complex biological matrices. In this context, liquid-chromatography coupled to high resolution mass spectrometry (LC-HRMS) is increasingly used and, via untargeted data-dependent MS/MS experiments, massive amounts of detailed information on the chemical composition of crude extracts can be generated. An efficient exploitation of such data sets requires automated data treatment and access to dedicated fragmentation databases. Various novel bioinformatics approaches such as molecular networking (MN) and in-silico fragmentation tools have emerged recently and provide new perspective for early metabolite identification in natural products (NPs) research. Here we propose an innovative dereplication strategy based on the combination of MN with an extensive in- silico MS/MS fragmentation database of NPs. Using two case studies, we demonstrate that this combined approach offers a powerful tool to navigate through the chemistry of complex NPs extracts, dereplicate metabolites, and annotate analogues of database entries.
DOI : 10.1021/acs.analchem.5b04804

L-F. Nothias-Scaglia, J-F. Gallard, V. Dumontet, F. Roussi, J. Costa, B. I. Iorga, J. Paolini, M. Litaudon. J. Nat. Prod. 2015, 78, 2423-2431.
Advanced Structural Determination of Diterpene Esters Using Molecular Modeling and NMR Spectroscopy. Three new jatrophane esters (1−3) were isolated from Euphorbia amygdaloides ssp. semiperfoliata, including an unprecedented macrocyclic jatrophane ester bearing a hemiketal substructure, named jatrohemiketal (3). The chemical structures of compounds 1−3 and their relative configurations were determined by spectroscopic analysis. The absolute configuration of compound 3 was determined unambiguously through an original strategy combining NMR spectroscopy and molecular modeling. Conformational search calculations were performed for the four possible diastereomers 3a−3d differing in their C-6 and C-9 stereocenters, and the lowest energy conformer was used as input structure for geometry optimization. The prediction of NMR parameters (1H and 13C chemical shifts and 1H−1H coupling constants) by density functional theory (DFT) calculations allowed identifying the most plausible diastereomer. Finally, the stereostructure of 3 was solved by comparison of the structural features obtained by molecular modeling for 3a−3d with NMR-derived data (the values of dihedral angles deduced from the vicinal proton−proton coupling constants (3 JHH) and interproton distances determined by ROESY). The methodology described herein provides an efficient way to solve or confirm structural elucidation of new macrocyclic diterpene esters, in particular when no crystal structure is available.
DOI : 10.1021/acs.jnatprod.5b00511

Correction to Advanced Structural Determination of Diterpene Esters Using Molecular Modeling and NMR Spectroscopy.
DOI : 10.1021/acs.jnatprod.5b01048
L-F. Nothias-Scaglia, I. Schmitz-Afonso, F. Renucci, F. Roussi, D. Touboul, J. Costa, M. Litaudon, J. Paolini. J. Chrom. A. 2015, 1422, 128-139.
Insights on profiling of phorbol, deoxyphorbol, ingenol and jatrophane diterpene esters by high performance liquid chromatography coupled to multiple stage mass spectrometry. This paper reports our effort to develop a comprehensive HPLC-MSn-based dereplication strategy for phorbol ester (PE), deoxyphorbol ester (dPE) and ingenol ester (IE) profiling in plant extracts. This strategy is composed of two sequential analysis exploiting specific hybrid triple quadrupole/linear ion trap instrument modes. A first run was performed using a multiple reaction monitoring (MRM) mode target- ing fragmentation of PE and dPE/IE coupled with the acquisition of MS2 spectrum for the ions at m/z 311 and m/z 313, respectively. A second run was then completed based on precursor ion scan mode (PIS) and automatic MS2 acquisition for each quasimolecular ion. The developed approach was used to investigate ten Euphorbia extracts showing bioactivity against chikungunya virus replication. Experiments allowed partial annotation of three dPE/IE but no PE was detected. Results suggested that other types of diterpene esters displayed PE- and dPE/IE-like fragmentations. The study of jatrophane ester (JE) standards by CID fragmentation using low and high resolution mass spectrometry confirmed this hypothesis, highlighting challenges and difficulties of diterpene esters profiling within plant extracts. Nonetheless, the present LC–MSn method can be easily adapted to profile other types of diterpene esters.
Développement méthodologique en synthèse organique
D. Brossard, P. Retailleau, V. Dumontet, P. Breton, S. Desrat, F. Roussi. Org. Biomol. Chem. 2017, 15, 5585-5592.
Fast & easy preparation of 3D scaffolds from methyl benzoate by a diversity oriented synthesis strategy based on Diels–Alder and ene-reactions. Thermic dimerization of methyl 1,3-cyclohexadiene 2-carboxylate gave original 3D-shape compounds by Diels–Alder cycloaddition and original [6 + 4]-ene reaction. Further selective modifications on an endo [4 + 2] cycloadduct via a diversity oriented synthesis (DOS) strategy quickly led to the preparation of a small library of original 3D scaffolds, providing access to a larger and unexplored chemical space for drug discovery processes.

S. Desrat, A. Ducousso, S. Gapil, C. Remeur, F. Roussi. Synlett 2015, 26, 385-387.
Methylaluminoxane (MAO)-Assisted Direct Amidation of Esters. Aliphatic and aromatic esters are efficiently transformed into amides in good to excellent yields, under mild conditions using methyl-aluminoxane (MAO). This reaction can be performed either at room temperature or by applying microwave irradiation.

Structures chimiques originales
M. Khazem, C. Jolly, T. Gaslonde, S. Massip, P. Negrier, P. Espeau, S. Michel, M. Litaudon. Eur. J. Org. Chem. 2018.
Spirokermeline : A Macrocyclic Spirolactone from Kermadecia elliptica Brongn. & Gris. Spirokermeline, a new macrocyclic compound derived from resorcinol, was isolated from Kermadecia elliptica, an endemic species of New Caledonia. The structure of spirokermeline was elucidated on the basis of spectroscopic analysis and X-ray single-crystal diffraction analysis. This is the first time that a 3H,3′H-spiro[benzofuran-2,1′-isobenzofuran]-3,3′-dione was isolated from higher plants and only the second time that this scaffold was found in nature. A possible biosynthetic pathway is proposed.
S. Remy, F. Olivon, S. Desrat, F. Blanchard, V. Eparvier, P. Leyssen, J. Neyts, F. Roussi, D. Touboul, M. Litaudon. J. Nat. Prod. 2018.
Structurally Diverse Diterpenoids from Sandwithia guyanensis. Bioassay-guided fractionation of an EtOAc extract of the trunk bark of Sandwithia guyanensis, using a chikungunya virus (CHIKV)-cell-based assay, afforded 17 new diterpenoids 1–17 and the known jatrointelones A and C (18 and 19). The new compounds included two tetranorditerpenoids 1 and 2, a trinorditerpenoid 3, euphoractines P-W (4–11), and euphactine G (13) possessing the rare 5/6/7/3 (4–7), 5/6/6/4 (8–11), and 5/6/8 (13) fused ring skeletons, sikkimenoid E (12), and jatrointelones J-M (14–17) possessing jatropholane and lathyrane carbon skeletons, respectively. Jatrointelones J (14) and M (17) represent the first naturally occurring examples of C-15 nonoxidized lathyrane-type diterpenoids. The structures of the new compounds were elucidated by NMR spectroscopic data analysis. The relative configuration of compound 16 and the absolute configurations of compounds 3–6 and 14 were determined by single-crystal X-ray diffraction analysis. In addition, jatrointelone K (15) was chemically transformed to euphoractine T (8) supporting the biosynthetic relationships between the two types of diterpenoids. Only compound 15 showed a moderate anti-CHIKV activity with an EC50 value of 14 μM. Finally, using a molecular networking-based dereplication strategy, several close analogues of 12-O-tetradecanoylphorbol-13-acetate (TPA), one of the most potent inhibitors of CHIKV replication, were dereplicated.
DOI : 10.1021/acs.jnatprod.7b01025

M. A. Beniddir, M-T. Martin, M-E. Tran Huu Dau, P. Rasoanaivo, F. Guéritte and M. Litaudon. Tetrahedron Lett. 2013, 54, 2115-2119.
Bisindole Alkaloid Artifacts from Gonioma Malagasy. Three new bisindole alkaloids (1–3), possessing a rare carbon skeleton formed by two indole moieties fused via a dihydropyran unit, were isolated from the alkaloid extract of the stem bark of Gonioma malagasy. Their structure and absolute configuration were assigned thanks to a combination of one and two dimensional NMR spectroscopy and ECD calculations. These bisindole alkaloids are artifacts formed during alkaloid extraction.
DOI : 10.1016/j.tetlet.2013.01.052


